Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
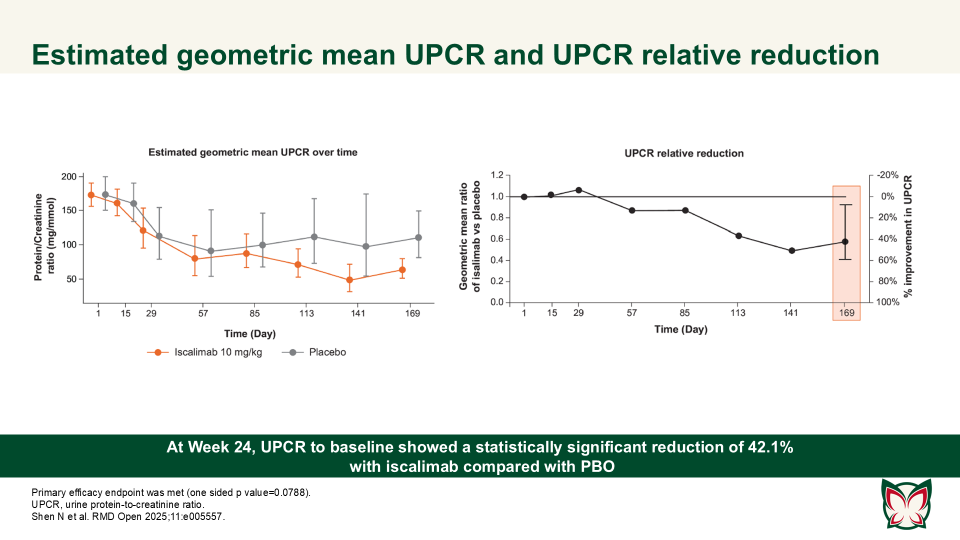
Efficacy, pharmacokinetics and safety of iscalimab (CFZ533) in patients with proliferative lupus nephritis: A randomised, double-blind, placebo-controlled, Phase II study
RMD Open 2025;11:e005557 Doi:10.1136/rmdopen-2025-005557
Shen N et al. report that iscalimab was clinically effective and generally well tolerated; in addition, it was devoid of the thromboembolic risk, characteristic of Fc active anti-CD40L therapies.
Outcomes of patients with systemic lupus erythematosus treated with belimumab: a post hoc efficacy analysis of five phase III clinical trials by British Isles Lupus Assessment Group-based Combined Lupus Assessment criteria
RMD Open 2025;11:e005-444 DOI:10.1136/rmdopen-2025-005444
Padrodis et al. aimed to determine belimumab efficacy assessed using BICLA in patients with SLE included in the phase III belimumab RCTs. The benefit of belimumab was found to be more prominent when combined with anti-malarial agents. Furthermore, using BICLA, the authors validated the results from foundational trials originally assessing belimumab efficacy using SLE Responder Index 4 thus, corroborating the efficacy of #belimumab in SLE.
Long-term effect of anifrolumab on patient-reported outcomes in systemic lupus erythematosus (TULIP-LTE): a randomised, placebo-controlled, phase 3 long-term extension trial
Lancet Rheumatol, 2025 DOI 10.1016/S2665-9913(25)00022-0
Strand et al. performed an exploratory analysis to assess patient-reported outcome measures, to investigate how patients with moderate-to-severe SLE perceive the effects of long-term treatment with #anifrolumab on their health status and health-related quality of life. They report improvements in health status and health-related quality of life, including differences favouring anifrolumab compared with placebo. These numerical improvements in patient- reported outcomes occurred alongside improvements in disease activity, reduced glucocorticoid doses, and a tolerable safety profile. These data suggest that anifrolumab is an effective treatment option that might improve health-related quality of life.
LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials
Ann Rheum Dis. 2025:S0003-496700071-8. DOI: 10.1016/j.ard.2025.01.016. Epub ahead of print
Morand et al. conducted a post-hoc analysis of the phase III TULIP-1 and TULIP-2 trials and their long-term extension, including 369 patients with moderate to severe SLE, to evaluate the long-term impact of anifrolumab on attainment of LLDAS and DORIS-defined remission. The results demonstrated that anifrolumab significantly improved the likelihood, speed, and duration of LLDAS and DORIS remission versus placebo over 4 years, with benefits sustained throughout the treatment period.
Keywords:
Opportunities and limitations of B bell depletion approaches in SLE
Nature Review Rheumatol, 2025;21:111–126 DOI: 10.1038/s41584-024-01210-9
Stockfelt et al. reviewed the long-term efficacy and challenges of B cell depletion strategies in SLE. Rituximab, a CD20-targeting monoclonal antibody, has demonstrated efficacy in a subset of patients but remains limited by immunogenicity, residual B cells, and B-cell activating factor (BAFF)-mediated relapse. Newer strategies incorporating CAR T cells, bispecific T cell engagers, and combination therapies aim to enhance B cell depletion and optimise outcomes.
Keywords:
Kidney Outcomes and Preservation of Kidney Function with Obinutuzumab in Patients with Lupus Nephritis: A Post Hoc Analysis of the NOBILITY Trial
Arthritis Rheumatol. 2023 DOI 10.1002/art.42734
This post hoc analysis of the Phase 2 NOBILITY trial determined that obinutuzumab plus standard of care improved the likelihood of long-term preservation of kidney function and improved kidney function with less glucocorticoid use in patients with lupus nephritis.
Keywords:
New Biologics and Targeted Therapies in Systemic Lupus: From New Molecular Targets to New Indications. A Systematic Review
Joint Bone Spine. 2023.
Systematic review of new biologics and targeted therapies in systemic lupus identifies a highly diversified pipeline of investigational drugs that will hopefully enable a more optimal treat-to-target strategy.
A Randomized, Placebo-Controlled Phase III Extension Trial of the Long-Term Safety and Tolerability of Anifrolumab in Active Systemic Lupus Erythematosus
Arthritis Rheumatol. 2022. Epub ahead of print doi: 10.1002/art.42392
Long-term extension study shows an acceptable long-term safety profile of anifrolumab in SLE, in addition to sustained improvements in disease activity and reduction in glucocorticoid use.