Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
Efficacy and safety of allogeneic CD19 CAR NK-cell therapy in systemic lupus erythematosus: A case series in China
Lancet 2025 Doi:10.1016/S0140-6736(25)01671-X Epub ahead of print
Gao et al. report that allogeneic CAR NK-cell therapy is a potent option for treatment of autoimmune diseases and may address limitations of current autologous CAR T-cell therapy, including manufacturing scale and time, access, safety, and cost. Authors evaluated the safety, tolerability, and efficacy of allogeneic CD19 CAR NK-cell therapy in patients with relapsed or refractory SLE.
Keywords:
Low-dose belimumab reduced risk of flares in patients with systemic lupus erythematosus: A multicentre, randomised, double-blind, placebo-controlled trial
Ann Rheum Dis 2025 Doi: 10.1016/j.ard.2025.10.010. Epub ahead of print
Sun et al. provides the first RCT evidence that low-dose belimumab reduces flares in patients with SLE with low-grade disease activity. Authors evaluated the efficacy of low-dose belimumab for disease flare prevention in Chinese patients with low-grade SLE (SELENA-SLEDAI ≤6).
Keywords:
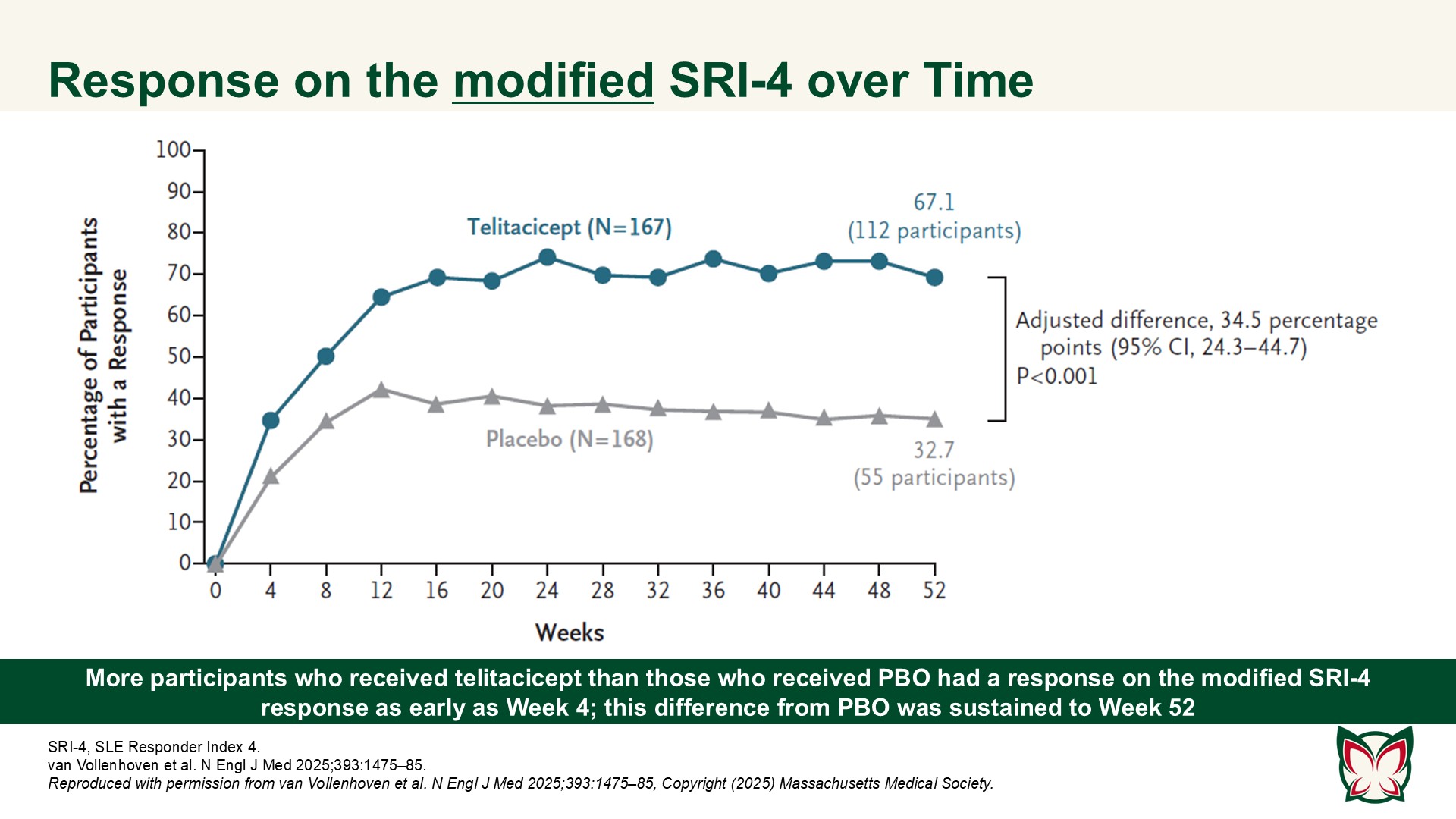
A Phase 3 trial of telitacicept for systemic lupus erythematosus
N Engl J Med 2025;393:1475-85 Doi: 10.1056/NEJMoa2414719
In this 52-week trial involving participants with active SLE who were receiving background therapy, the incidence of a clinical response was higher with telitacicept than with PBO. van Vollenhoven et al. report efficacy and safety results of a Phase 3 trial of telitacicept at a dose of 160mg weekly as compared with PBO in Chinese persons with active SLE.
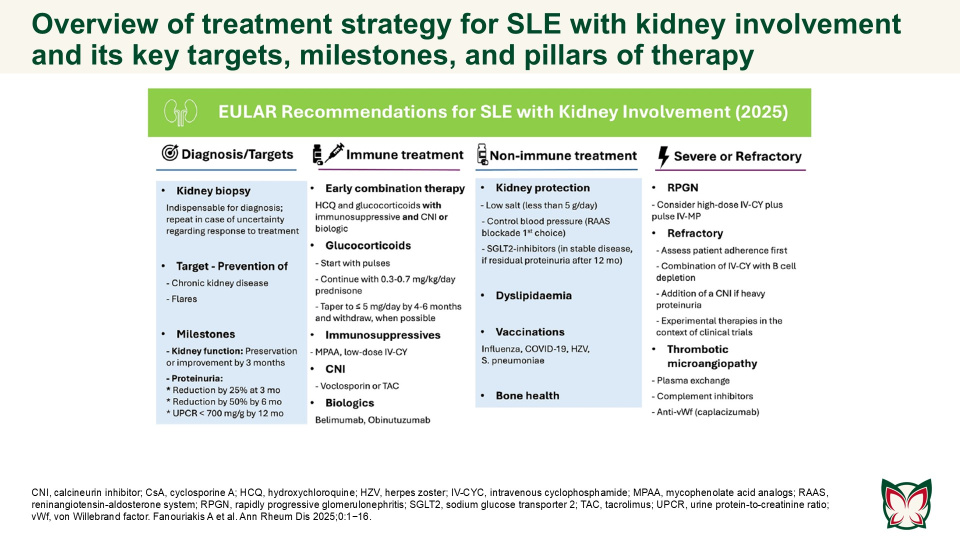
EULAR recommendations for the management of systemic lupus erythematosus with kidney involvement: 2025 update
Ann Rheum Dis 2025;0:1−16 Doi: 10.1016/j.ard.2025.09.007
The updated EULAR recommendations provide evidence- and expert-based consensus on the management of SLE with kidney involvement, adjusted for severity, and taking into consideration long-term efficacy, safety, cost, and local availability of drugs. Fanouriakis A et al. updated the 2019 EULAR/ ERA-EDTA recommendations for the management of SLE with kidney involvement, taking into consideration emerging evidence and recent developments in the field.
Keywords:
Secukinumab in active lupus nephritis: Results from Phase III, randomised, placebo controlled study (SELUNE) and open-label extension study
Rheumatology 2025 Doi: 10.1093/rheumatology/keaf536 Epub ahead of print
Secukinumab did not demonstrate superior efficacy over PBO in patients with active LN; secukinumab was well-tolerated with no new or unexpected safety signals detected. A Phase-III core study (SELUNE) and an extension study, were conducted by Zhao et al. to evaluate the efficacy and safety of SC secukinumab 300mg compared with PBO, in combination with the SoC, in patients with active LN.
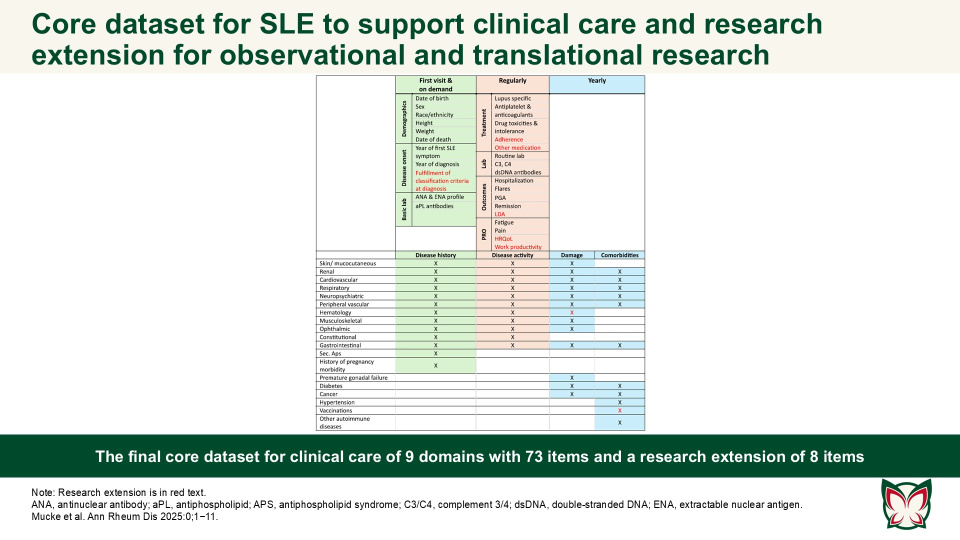
EULAR recommendations for a core dataset to support clinical care and translational and observational research in systemic lupus erythematosus
Ann Rheum Dis 2025:0;1−11 Doi: 10.1016/j.ard.2025.07.001
The presented clinical core dataset and its research extension are designed to improve SLE patient care and facilitate collaborative research by ensuring the comparability of datasets and cohort descriptions. The aim of this EULAR taskforce by Mucke et al. was to define a core set of essential items for the comprehensive care of SLE patients in clinical practice, with an extension for vital elements required for translational and observational research.
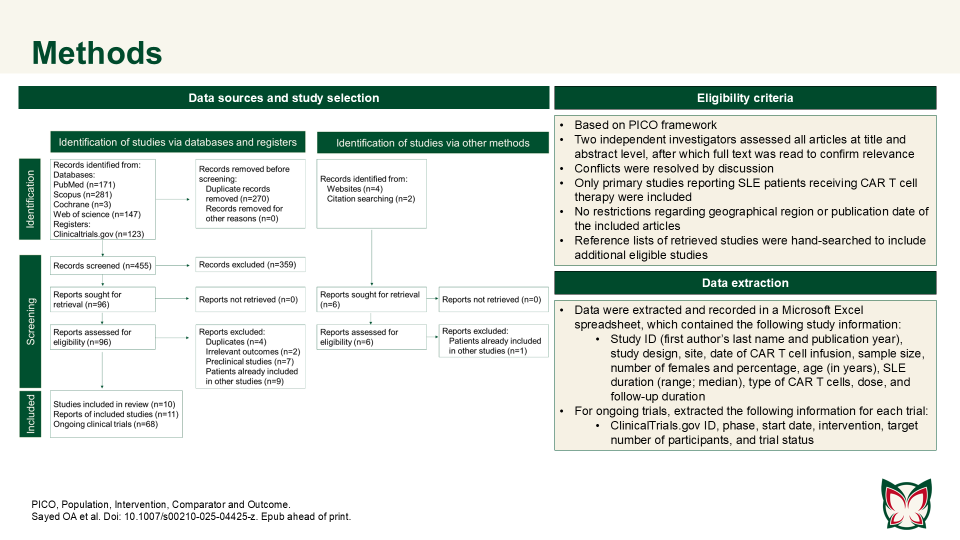
CAR T cell therapy efficacy and safety in SLE: A systematic review and pooled analysis of 47 patients across 10 studies
Doi: 10.1007/s00210-025-04425-z
Sayed OA et al. reported that CAR T cell therapy showed promise in refractory SLE, achieving durable remission with manageable toxicity; however further trials are needed to confirm long-term outcomes. Authors consolidated the current evidence on CAR T cell therapy in the treatment of SLE.
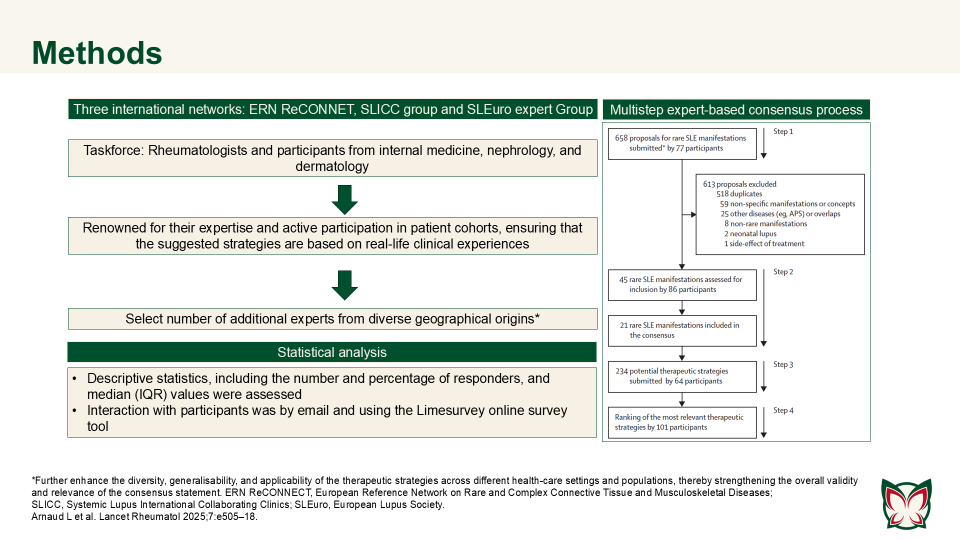
ERN ReCONNET–SLICC–SLEuro expert consensus on the therapeutic management of rare systemic lupus erythematosus manifestations
Lancet Rheumatol 2025;7:e505–18 Doi: 10.1016/S2665-9913(25)00063-3
This systematic review highlighted the major gaps in evidence regarding rare SLE manifestations, with many findings dependent on variable methodologies or single reports. Arnard et al. established an international consensus on therapeutic strategies for rare SLE manifestations.
Keywords:
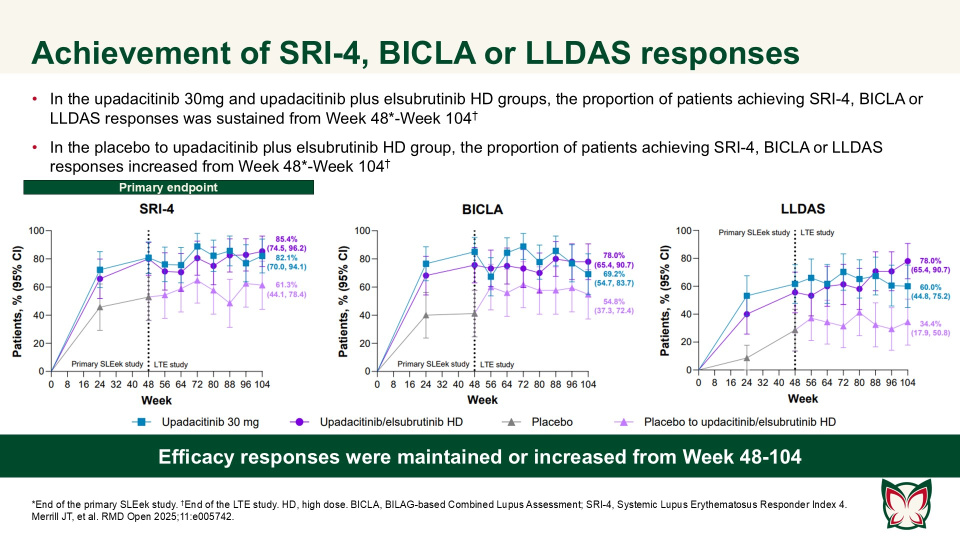
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
Deucravacitinib, an oral, selective, allosteric, tyrosine kinase 2 inhibitor, in patients with active SLE: efficacy on patient-reported outcomes in a phase II randomised trial
Lupus Sci Med. 2025;12(1):e001517
Patient-reported outcomes from the deucravacitinib, 48-week, phase II, PAISLEY study show that patients with SLE experienced greater improvements in pain, fatigue and health-related quality-of-life scores at Week 48 with deucravacitinib versus placebo treatment.