Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
Efficacy and safety of allogeneic CD19 CAR NK-cell therapy in systemic lupus erythematosus: A case series in China
Lancet 2025 Doi:10.1016/S0140-6736(25)01671-X Epub ahead of print
Gao et al. report that allogeneic CAR NK-cell therapy is a potent option for treatment of autoimmune diseases and may address limitations of current autologous CAR T-cell therapy, including manufacturing scale and time, access, safety, and cost. Authors evaluated the safety, tolerability, and efficacy of allogeneic CD19 CAR NK-cell therapy in patients with relapsed or refractory SLE.
Keywords:
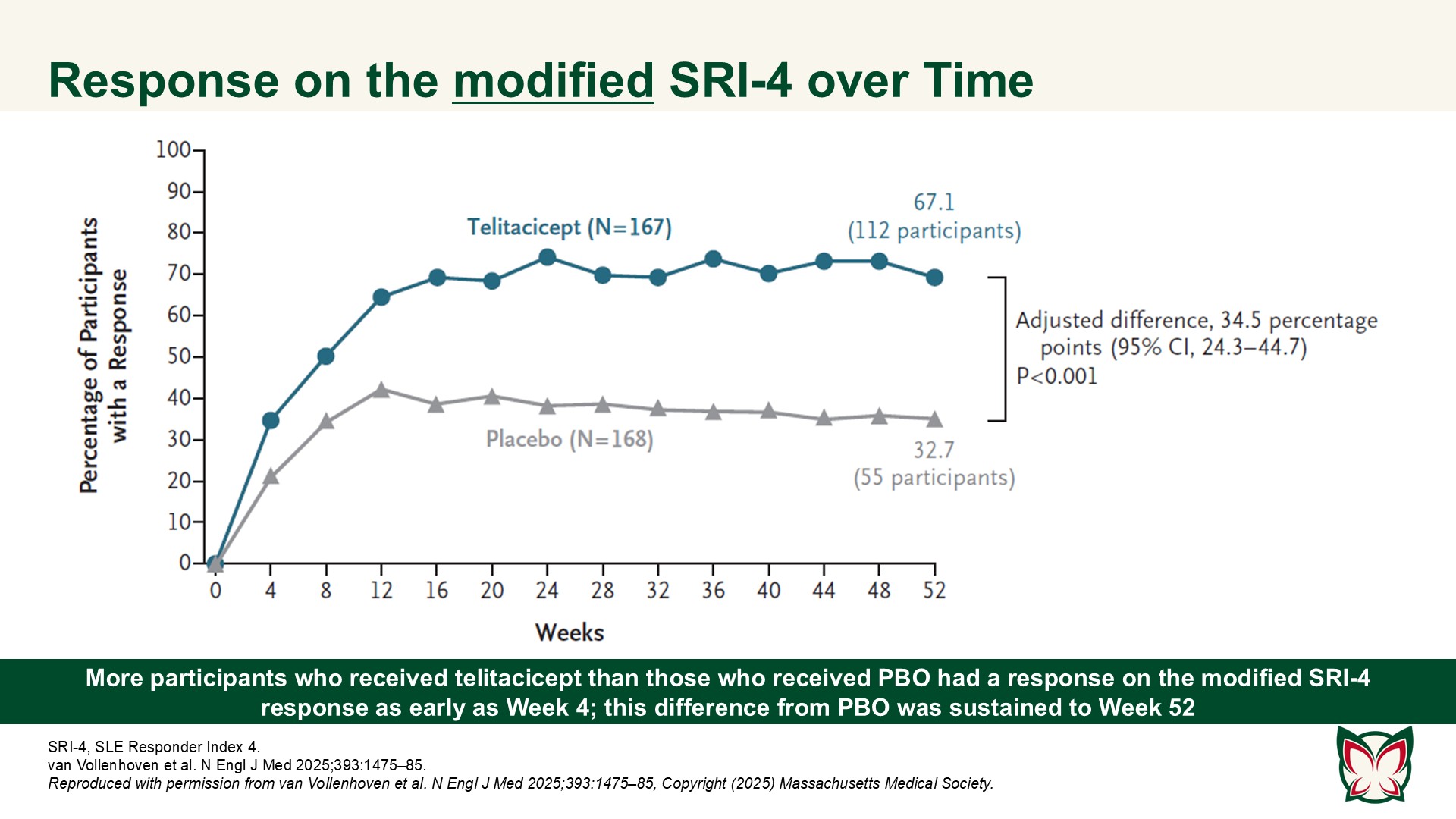
A Phase 3 trial of telitacicept for systemic lupus erythematosus
N Engl J Med 2025;393:1475-85 Doi: 10.1056/NEJMoa2414719
In this 52-week trial involving participants with active SLE who were receiving background therapy, the incidence of a clinical response was higher with telitacicept than with PBO. van Vollenhoven et al. report efficacy and safety results of a Phase 3 trial of telitacicept at a dose of 160mg weekly as compared with PBO in Chinese persons with active SLE.
Telitacicept in Patients with Active Systemic Lupus Erythematosus: Results of A Phase 2b, Randomised, Double-blind, Placebo-controlled Trial
Ann Rheum Dis. 2023; DOI: 10.1136/ard-2023-224854
This Phase 2 trial demonstrated the efficacy and acceptable safety profile of telitacicept in patients with SLE. The safety profile of telitacicept was comparable with that observed in clinical trials of other B cell-targeting agents.