Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
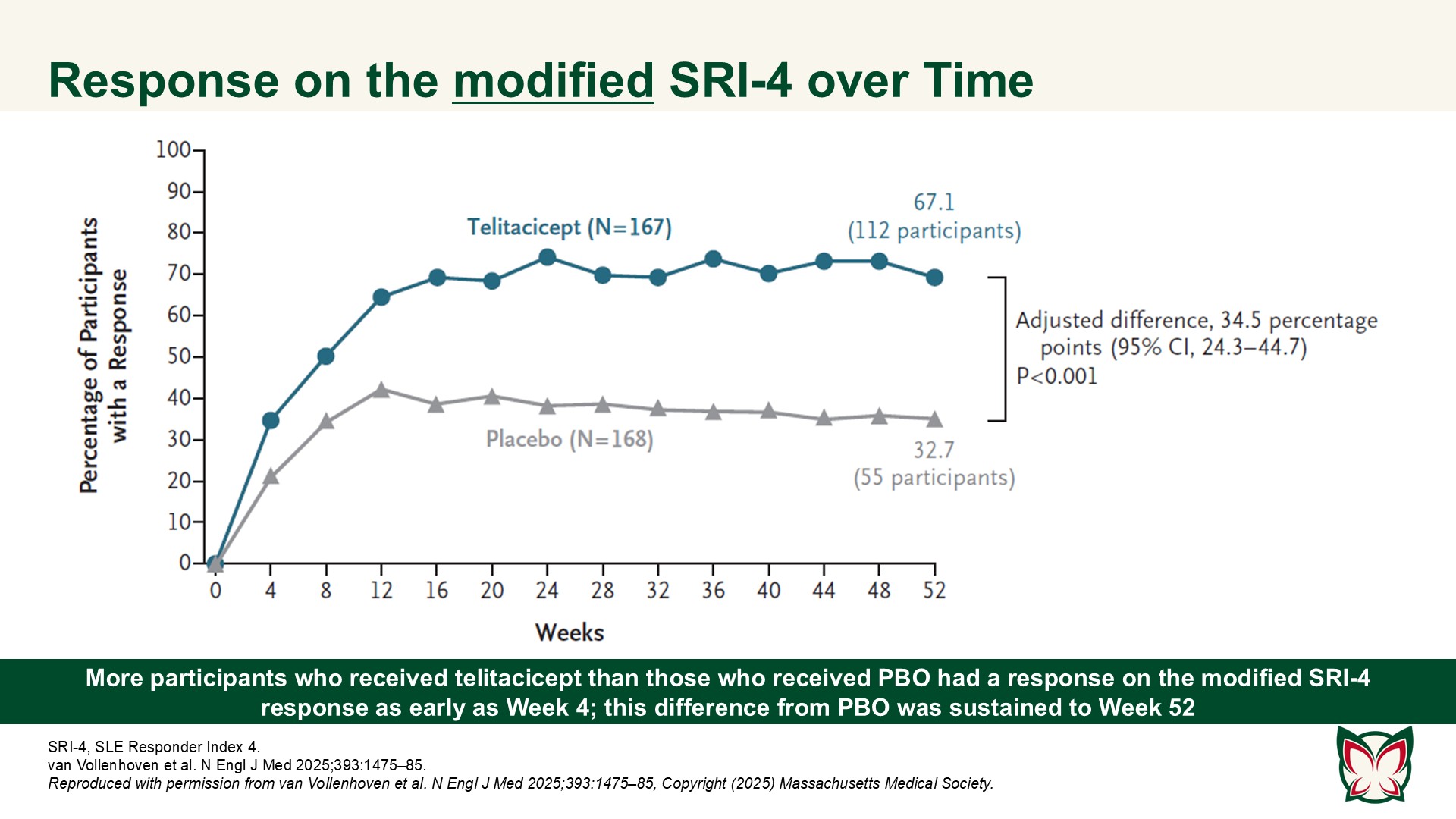
A Phase 3 trial of telitacicept for systemic lupus erythematosus
N Engl J Med 2025;393:1475-85 Doi: 10.1056/NEJMoa2414719
In this 52-week trial involving participants with active SLE who were receiving background therapy, the incidence of a clinical response was higher with telitacicept than with PBO. van Vollenhoven et al. report efficacy and safety results of a Phase 3 trial of telitacicept at a dose of 160mg weekly as compared with PBO in Chinese persons with active SLE.
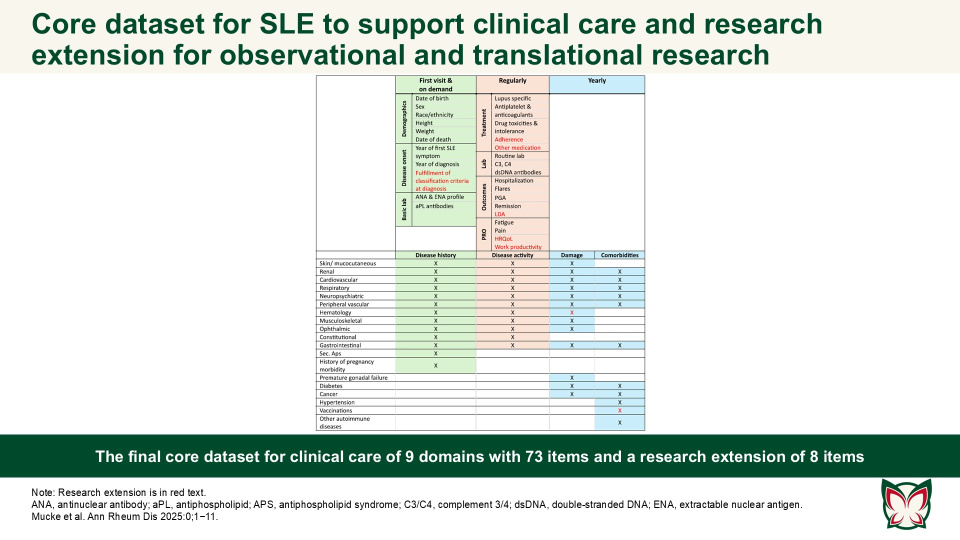
EULAR recommendations for a core dataset to support clinical care and translational and observational research in systemic lupus erythematosus
Ann Rheum Dis 2025:0;1−11 Doi: 10.1016/j.ard.2025.07.001
The presented clinical core dataset and its research extension are designed to improve SLE patient care and facilitate collaborative research by ensuring the comparability of datasets and cohort descriptions. The aim of this EULAR taskforce by Mucke et al. was to define a core set of essential items for the comprehensive care of SLE patients in clinical practice, with an extension for vital elements required for translational and observational research.
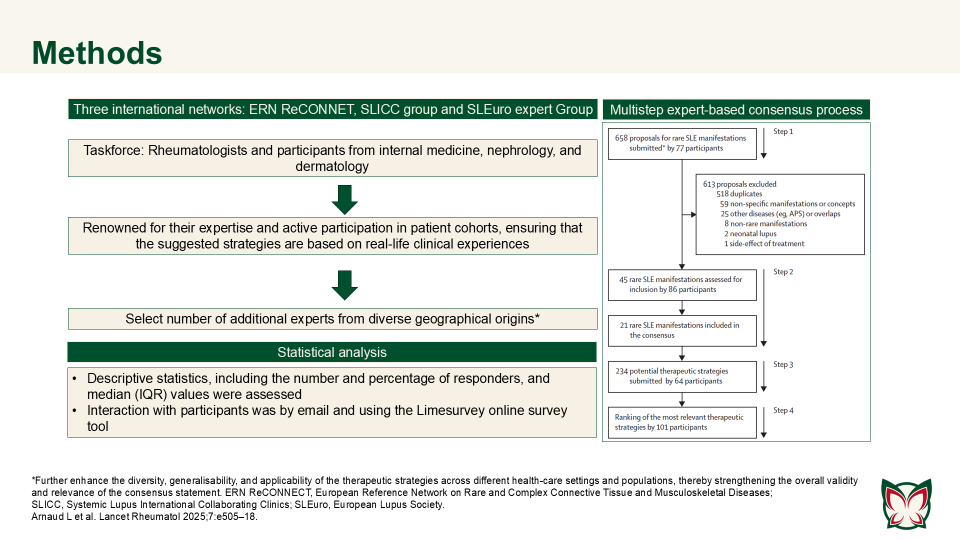
ERN ReCONNET–SLICC–SLEuro expert consensus on the therapeutic management of rare systemic lupus erythematosus manifestations
Lancet Rheumatol 2025;7:e505–18 Doi: 10.1016/S2665-9913(25)00063-3
This systematic review highlighted the major gaps in evidence regarding rare SLE manifestations, with many findings dependent on variable methodologies or single reports. Arnard et al. established an international consensus on therapeutic strategies for rare SLE manifestations.
Keywords:
Domains for inclusion in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE): results of a modified Delphi study
Lupus Sci Med. 2025 May 6;12(1):e001484 doi: 10.1136/lupus-2024-001484
Connelly, et al. use Delphi methods to achieve consensus to include eight domains of active disease to define treatment response in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE).
Outcomes of patients with systemic lupus erythematosus treated with belimumab: a post hoc efficacy analysis of five phase III clinical trials by British Isles Lupus Assessment Group-based Combined Lupus Assessment criteria
RMD Open 2025;11:e005-444 DOI:10.1136/rmdopen-2025-005444
Padrodis et al. aimed to determine belimumab efficacy assessed using BICLA in patients with SLE included in the phase III belimumab RCTs. The benefit of belimumab was found to be more prominent when combined with anti-malarial agents. Furthermore, using BICLA, the authors validated the results from foundational trials originally assessing belimumab efficacy using SLE Responder Index 4 thus, corroborating the efficacy of #belimumab in SLE.
LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials
Ann Rheum Dis. 2025:S0003-496700071-8. DOI: 10.1016/j.ard.2025.01.016. Epub ahead of print
Morand et al. conducted a post-hoc analysis of the phase III TULIP-1 and TULIP-2 trials and their long-term extension, including 369 patients with moderate to severe SLE, to evaluate the long-term impact of anifrolumab on attainment of LLDAS and DORIS-defined remission. The results demonstrated that anifrolumab significantly improved the likelihood, speed, and duration of LLDAS and DORIS remission versus placebo over 4 years, with benefits sustained throughout the treatment period.
Keywords:
Effect of iberdomide on cutaneous manifestations in systemic lupus erythematosus: a randomized phase 2 clinical trial
JAAD. 2024. Epub ahead of print DOI: 10.1016/j.jaad.2024.09.074
Werth et al. demonstrated that iberdomide significantly improved cutaneous lupus erythematosus (CLE) outcomes, particularly in subacute and chronic CLE patients, by reducing Cutaneous Lupus Area and Severity Index Activity (CLASI-A) scores. The study showed continued efficacy through 24 weeks, with the 0.45 mg dose providing the greatest improvement in patients with severe baseline scores, and iberdomide was well-tolerated over 104 weeks.
Keywords:
Efficacy and safety of sequential therapy with subcutaneous belimumab and one cycle of rituximab in patients with systemic lupus erythematosus: the phase 3, randomised, placebo-controlled BLISS-BELIEVE study
Ann Rheum Dis 2024;0:1–11 DOI 10.1136/ard-2024-225686.
Aranow et al. evaluated the efficacy and safety of combining subcutaneous belimumab with one cycle of rituximab in SLE. Sequential therapy did not show a statistically significant improvement in disease control over belimumab monotherapy, but did achieve nominally better reductions in disease activity markers.
Keywords:
Telitacicept in Patients with Active Systemic Lupus Erythematosus: Results of A Phase 2b, Randomised, Double-blind, Placebo-controlled Trial
Ann Rheum Dis. 2023; DOI: 10.1136/ard-2023-224854
This Phase 2 trial demonstrated the efficacy and acceptable safety profile of telitacicept in patients with SLE. The safety profile of telitacicept was comparable with that observed in clinical trials of other B cell-targeting agents.
Keywords:
Association Between Severe Nonadherence to Hydroxychloroquine and Systemic Lupus Erythematosus Flares, Damage, and Mortality in 660 Patients From the SLICC Inception Cohort
Arthritis Rheumatol. 2023; 75(12):2195–2206 DOI: 10.1002/art.42645
n this study, severe nonadherence to hydroxychloroquine (HCQ) was independently associated with the risk of SLE flare in the following year, early damage and 5-year mortality.