A Phase 3 trial of telitacicept for systemic lupus erythematosus
N Engl J Med 2025;393:1475-85 Doi: 10.1056/NEJMoa2414719
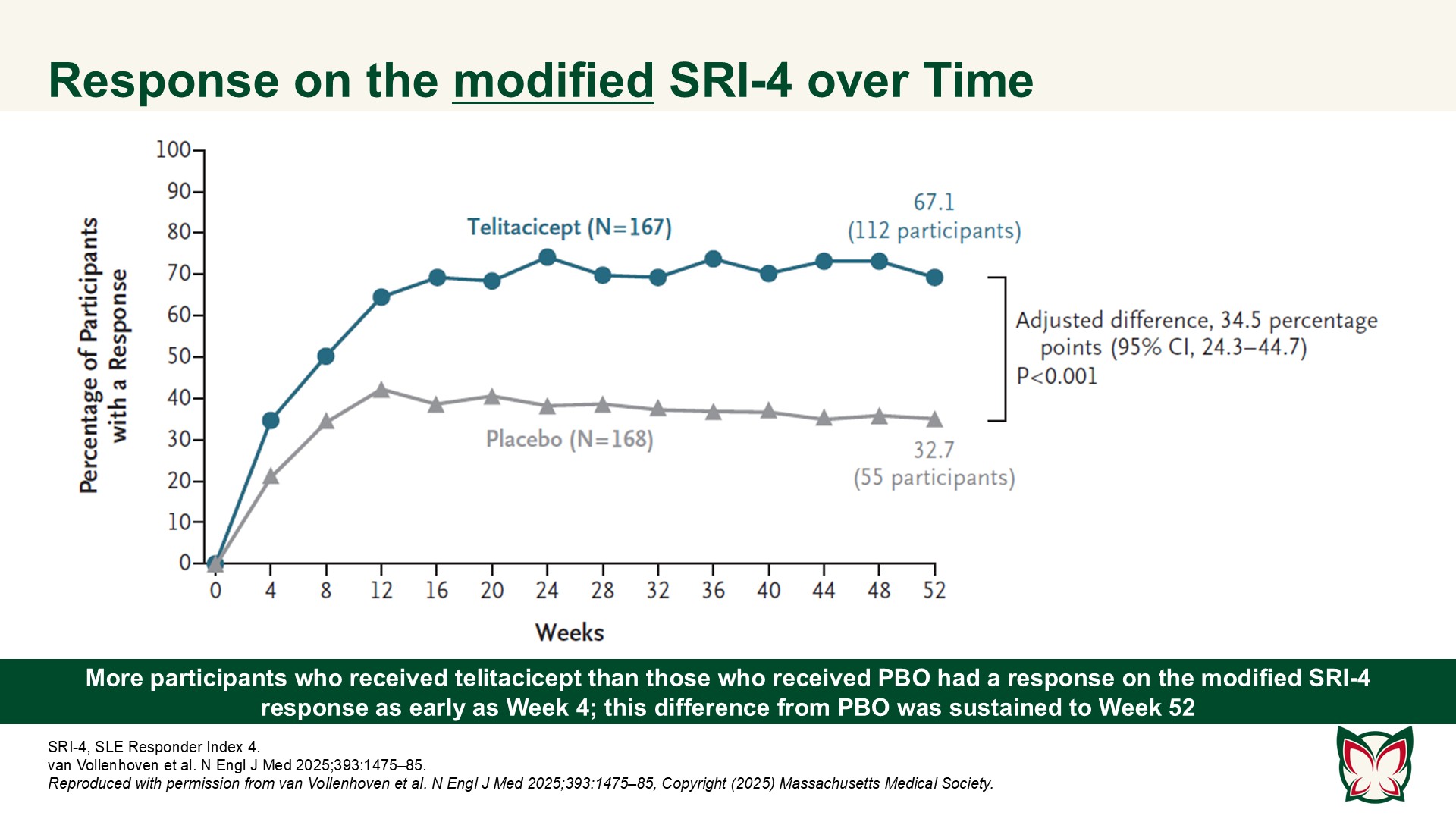
In this 52-week trial involving participants with active SLE who were receiving background therapy, the incidence of a clinical response was higher with telitacicept than with PBO. van Vollenhoven et al. report efficacy and safety results of a Phase 3 trial of telitacicept at a dose of 160mg weekly as compared with PBO in Chinese persons with active SLE.
The results of this Phase 3 trial showed the efficacy of telitacicept in Chinese persons with SLE. Although there was no signal of increased SAEs as compared with PBO, telitacicept was associated with increased upper respiratory infections, decreases in immunoglobulin levels, and increased injection-site reactions.