Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
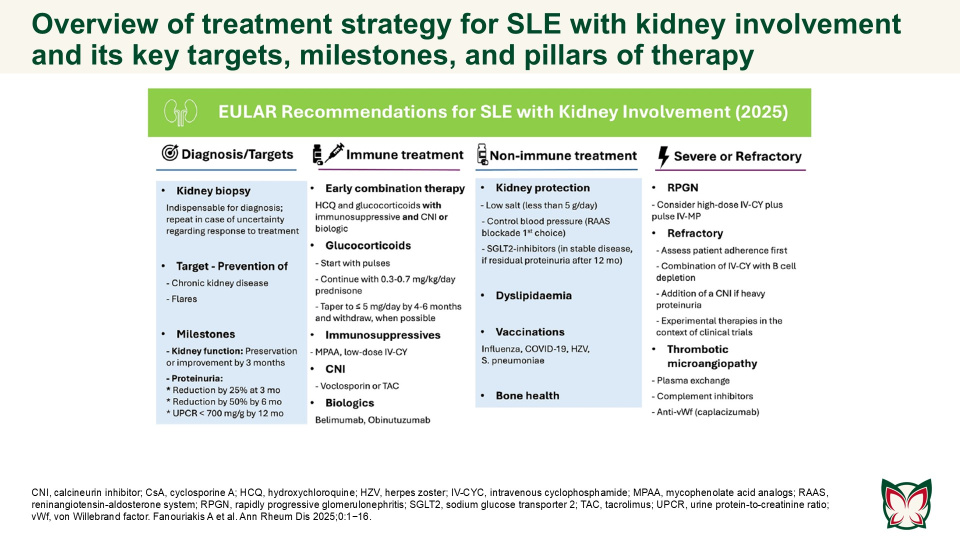
EULAR recommendations for the management of systemic lupus erythematosus with kidney involvement: 2025 update
Ann Rheum Dis 2025;0:1−16 Doi: 10.1016/j.ard.2025.09.007
The updated EULAR recommendations provide evidence- and expert-based consensus on the management of SLE with kidney involvement, adjusted for severity, and taking into consideration long-term efficacy, safety, cost, and local availability of drugs. Fanouriakis A et al. updated the 2019 EULAR/ ERA-EDTA recommendations for the management of SLE with kidney involvement, taking into consideration emerging evidence and recent developments in the field.
Keywords:
Domains for inclusion in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE): results of a modified Delphi study
Lupus Sci Med. 2025 May 6;12(1):e001484 doi: 10.1136/lupus-2024-001484
Connelly, et al. use Delphi methods to achieve consensus to include eight domains of active disease to define treatment response in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE).
LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials
Ann Rheum Dis. 2025:S0003-496700071-8. DOI: 10.1016/j.ard.2025.01.016. Epub ahead of print
Morand et al. conducted a post-hoc analysis of the phase III TULIP-1 and TULIP-2 trials and their long-term extension, including 369 patients with moderate to severe SLE, to evaluate the long-term impact of anifrolumab on attainment of LLDAS and DORIS-defined remission. The results demonstrated that anifrolumab significantly improved the likelihood, speed, and duration of LLDAS and DORIS remission versus placebo over 4 years, with benefits sustained throughout the treatment period.
Keywords:
Efficacy and safety of obinutuzumab in active lupus nephritis
NEJM, 2025. Epub ahead of print. DOI: 10.1056/NEJMoa2410965
Furie et al. demonstrated that obinutuzumab plus standard therapy significantly improved complete renal response at Wk76 compared with placebo. No unexpected safety signals were identified, though infections and COVID-19-related events were more frequent in the obinutuzumab group.
Effect of iberdomide on cutaneous manifestations in systemic lupus erythematosus: a randomized phase 2 clinical trial
JAAD. 2024. Epub ahead of print DOI: 10.1016/j.jaad.2024.09.074
Werth et al. demonstrated that iberdomide significantly improved cutaneous lupus erythematosus (CLE) outcomes, particularly in subacute and chronic CLE patients, by reducing Cutaneous Lupus Area and Severity Index Activity (CLASI-A) scores. The study showed continued efficacy through 24 weeks, with the 0.45 mg dose providing the greatest improvement in patients with severe baseline scores, and iberdomide was well-tolerated over 104 weeks.
Keywords:
Efficacy and safety of sequential therapy with subcutaneous belimumab and one cycle of rituximab in patients with systemic lupus erythematosus: the phase 3, randomised, placebo-controlled BLISS-BELIEVE study
Ann Rheum Dis 2024;0:1–11 DOI 10.1136/ard-2024-225686.
Aranow et al. evaluated the efficacy and safety of combining subcutaneous belimumab with one cycle of rituximab in SLE. Sequential therapy did not show a statistically significant improvement in disease control over belimumab monotherapy, but did achieve nominally better reductions in disease activity markers.
Keywords:
Type I interferon blockade with anifrolumab in patients with systemic lupus erythematosus modulates key immunopathological pathways in a gene expression and proteomic analysis of two Phase 3 trials
Ann Rheum Dis 2024 DOI 10.1136/ard-2023-225445 Epub ahead of print https://pubmed.ncbi.nlm.nih.gov/38569851/
Type I IFN blockade with anifrolumab modulated multiple inflammatory pathways downstream of type I IFN signalling.
Kidney Outcomes and Preservation of Kidney Function with Obinutuzumab in Patients with Lupus Nephritis: A Post Hoc Analysis of the NOBILITY Trial
Arthritis Rheumatol. 2023 DOI 10.1002/art.42734
This post hoc analysis of the Phase 2 NOBILITY trial determined that obinutuzumab plus standard of care improved the likelihood of long-term preservation of kidney function and improved kidney function with less glucocorticoid use in patients with lupus nephritis.
Keywords:
Burden of Systemic Lupus Erythematosus in Clinical Practice: Baseline Data from the SLE Prospective Observational Cohort Study (SPOCS) by Interferon Gene Signature
Lupus Sci Med. 2023; 10(2):e001032 DOI: 10.1136/lupus-2023-001032
This study from Arnaud et al described baseline characteristics of SLE patients grouped by disease activity and IFNGS category in the international SPOCS study. IFNGS-high patients were younger at SLE diagnosis, and a baseline SLEDAI-2K score ≥10 was associated with shorter disease duration, more frequent and more severe flares. IFNGS-low patients were more likely to exhibit musculoskeletal and CNS comorbidities than IFNGS-high patients. Continuation of the SPOCS study will allow investigation into how different baseline characteristics affect long-term outcomes in SLE patients.
Keywords:
EULAR Recommendations for the Management of Systemic Lupus Erythematosus: 2023 Update
Ann Rheum Dis 2023;83(1):15–29 DOI: 10.1136/ard-2023-224762
The objective of this international task force was to update the EULAR recommendations for the management of SLE. The Task Force agreed on 5 overarching principles and 13 recommendations, generating an overall framework for the approach to a patient with SLE. The updated recommendations provide consensus guidance on the management of SLE, combining evidence and expert opinion.