Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
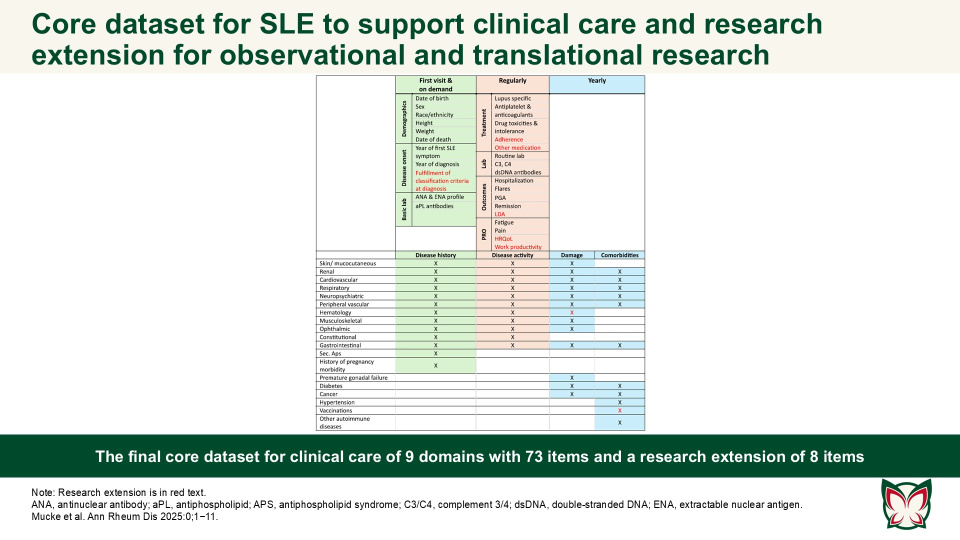
EULAR recommendations for a core dataset to support clinical care and translational and observational research in systemic lupus erythematosus
Ann Rheum Dis 2025:0;1−11 Doi: 10.1016/j.ard.2025.07.001
The presented clinical core dataset and its research extension are designed to improve SLE patient care and facilitate collaborative research by ensuring the comparability of datasets and cohort descriptions. The aim of this EULAR taskforce by Mucke et al. was to define a core set of essential items for the comprehensive care of SLE patients in clinical practice, with an extension for vital elements required for translational and observational research.
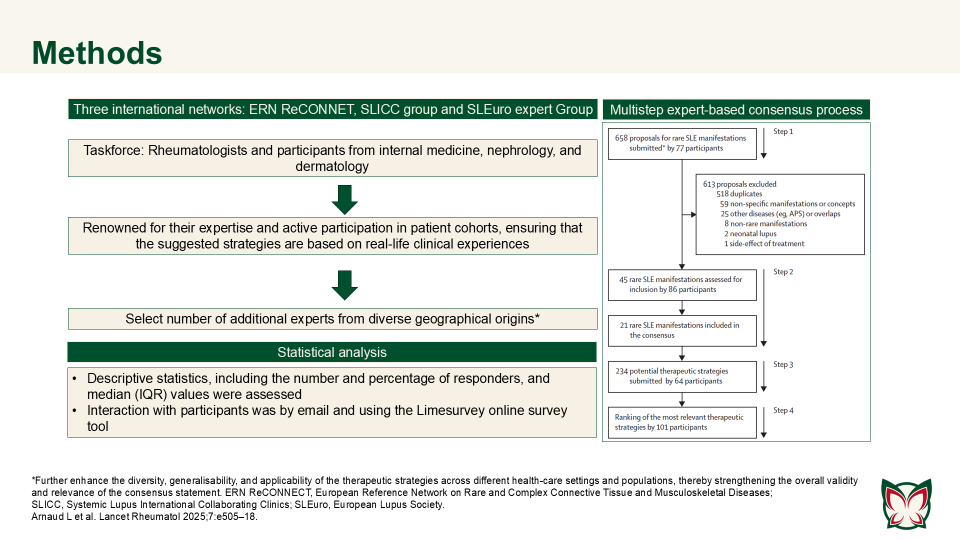
ERN ReCONNET–SLICC–SLEuro expert consensus on the therapeutic management of rare systemic lupus erythematosus manifestations
Lancet Rheumatol 2025;7:e505–18 Doi: 10.1016/S2665-9913(25)00063-3
This systematic review highlighted the major gaps in evidence regarding rare SLE manifestations, with many findings dependent on variable methodologies or single reports. Arnard et al. established an international consensus on therapeutic strategies for rare SLE manifestations.
Keywords:
Domains for inclusion in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE): results of a modified Delphi study
Lupus Sci Med. 2025 May 6;12(1):e001484 doi: 10.1136/lupus-2024-001484
Connelly, et al. use Delphi methods to achieve consensus to include eight domains of active disease to define treatment response in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE).
Evolution and trajectory of B-cell targeted therapies in rheumatic diseases
Lancet Rheumatol, 2025. Epub ahead of print
Carter et al. review the clinical and mechanistic development of B-cell targeted therapies over the last two decades in autoimmune rheumatic diseases. B-cell depletion depth, repopulation dynamics, and immunogenicity determine long-term efficacy and inform the rationale for emerging
dual-targeted approaches, particularly in systemic lupus erythematosus where belimumab and rituximab combinations show potential to mitigate relapse driven by BAFF.
Opportunities and limitations of B bell depletion approaches in SLE
Nature Review Rheumatol, 2025;21:111–126 DOI: 10.1038/s41584-024-01210-9
Stockfelt et al. reviewed the long-term efficacy and challenges of B cell depletion strategies in SLE. Rituximab, a CD20-targeting monoclonal antibody, has demonstrated efficacy in a subset of patients but remains limited by immunogenicity, residual B cells, and B-cell activating factor (BAFF)-mediated relapse. Newer strategies incorporating CAR T cells, bispecific T cell engagers, and combination therapies aim to enhance B cell depletion and optimise outcomes.
Keywords:
ANA-associated arthritis: clinical and biomarker characterization of a population for basket trials
Rheumatol 2024 2024;00:1–11 DOI 10.1093/rheumatology/keae269
Arnold et al. assessed musculoskeletal (MSK) inflammation in ANA-associated rheumatic diseases (RMDs) and redefined ANA-associated arthritis into two distinct multi-disease clusters based on disease activity, which may support a more targeted approach to treatment. The authors confirmed that MSK inflammation is a key feature across diagnoses and responded similarly to treatments.
Keywords:
Type I interferon blockade with anifrolumab in patients with systemic lupus erythematosus modulates key immunopathological pathways in a gene expression and proteomic analysis of two Phase 3 trials
Ann Rheum Dis 2024 DOI 10.1136/ard-2023-225445 Epub ahead of print https://pubmed.ncbi.nlm.nih.gov/38569851/
Type I IFN blockade with anifrolumab modulated multiple inflammatory pathways downstream of type I IFN signalling.
EULAR Recommendations for the Management of Systemic Lupus Erythematosus: 2023 Update
Ann Rheum Dis 2023;83(1):15–29 DOI: 10.1136/ard-2023-224762
The objective of this international task force was to update the EULAR recommendations for the management of SLE. The Task Force agreed on 5 overarching principles and 13 recommendations, generating an overall framework for the approach to a patient with SLE. The updated recommendations provide consensus guidance on the management of SLE, combining evidence and expert opinion.
Keywords:
Rapid Efficacy of Anifrolumab Across Multiple Subtypes of Recalcitrant Cutaneous Lupus Erythematosus Parallels Changes in Discrete Subsets of Blood Transcriptomic and Cellular Biomarkers
Br J Dermatol. 2023 doi: 10.1093/bjd/ljad089
Prospective single-centre study of anifrolumab in refractory mucocutaneous SLE, indicates rapid efficacy of anifrolumab in discoid lupus erythematosus (DLE) and rituximab-resistant CLE.
Keywords:
2022 EULAR points to consider for the measurement, reporting and application of IFN-I pathway activation assays in clinical research and practice
Ann Rheum Dis. 2023 doi: 10.1136/ard-2022-223628
The first EULAR-endorsed points to consider (PtC) for the measurement and reporting of IFN-I assays in clinical research and practice are developed.