Secukinumab in active lupus nephritis: Results from Phase III, randomised, placebo controlled study (SELUNE) and open-label extension study
Rheumatology 2025 Doi: 10.1093/rheumatology/keaf536 Epub ahead of print
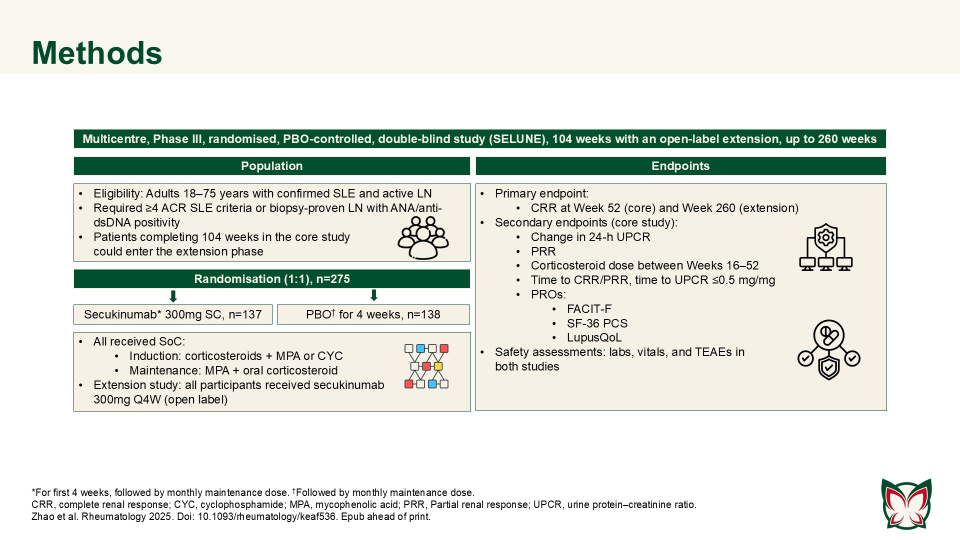
Secukinumab did not demonstrate superior efficacy over PBO in patients with active LN; secukinumab was well-tolerated with no new or unexpected safety signals detected. A Phase-III core study (SELUNE) and an extension study, were conducted by Zhao et al. to evaluate the efficacy and safety of SC secukinumab 300mg compared with PBO, in combination with the SoC, in patients with active LN.
Both studies were terminated early due to futile results following a planned futility analysis of the core study. Secukinumab combined with SoC did not demonstrate superior efficacy versus PBO with SoC in patients with active LN. The safety profile of secukinumab was consistent with the known safety profile in approved adult indications and showed no new or unexpected safety signals in patients with LN.