Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
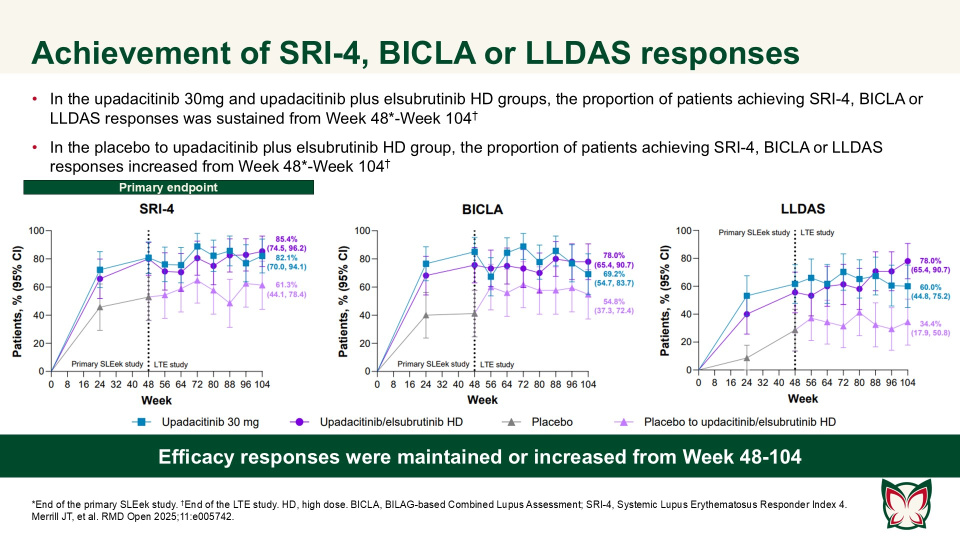
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
Belimumab efficacy in mucocutaneous lupus erythematosus: a large post-hoc analysis from five phase III clinical trials
Rheumatol, 2025. Epub ahead of print. DOI: 10.1093/rheumatology/keaf145
Grosso et al. conducted a post-hoc analysis of five phase III trials, including 3086 patients, to evaluate the efficacy of belimumab in improving mucocutaneous manifestations of SLE. The results demonstrated that belimumab significantly improved disease activity as measured by the mcBILAG and mcSLEDAI-2K indices compared with placebo and reduced flare rates in patients with high disease activity and serological positivity.
Keywords:
Early Infection Risk in Patients with Systemic Lupus Erythematosus Treated with Rituximab or Belimumab from the British Isles Lupus Assessment Group Biologics Register (BILAG-BR): A Prospective Longitudinal Study
Lancet Rheumatol 2023;5:e284–92 doi:https://doi.org/10.1016/S2665-9913(23)00091-7
Data from a large prospective registry (BILAG-BR) highlight that, compared with standard of care, the serious infection risk was similar between rituximab and belimumab.
Keywords:
Easy-BILAG: a new tool for simplified recording of SLE disease activity using BILAG-2004 index
Rheumatology (Oxford). 2022. Epub ahead of print doi: 10.1093/rheumatology/keab883
Easy-BILAG is a high-accuracy, time-efficient tool for recording BILAG-2004 disease activity in systemic lupus erythematosus (SLE).
Disease activity measurements in SLE are necessary for optimal patient care, treat-to-target approaches and clinical guidelines. However, administrative burden and potential frequency of errors with the current comprehensive disease activity instrument (BILAG-2004) limits its use in routine practice.