Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
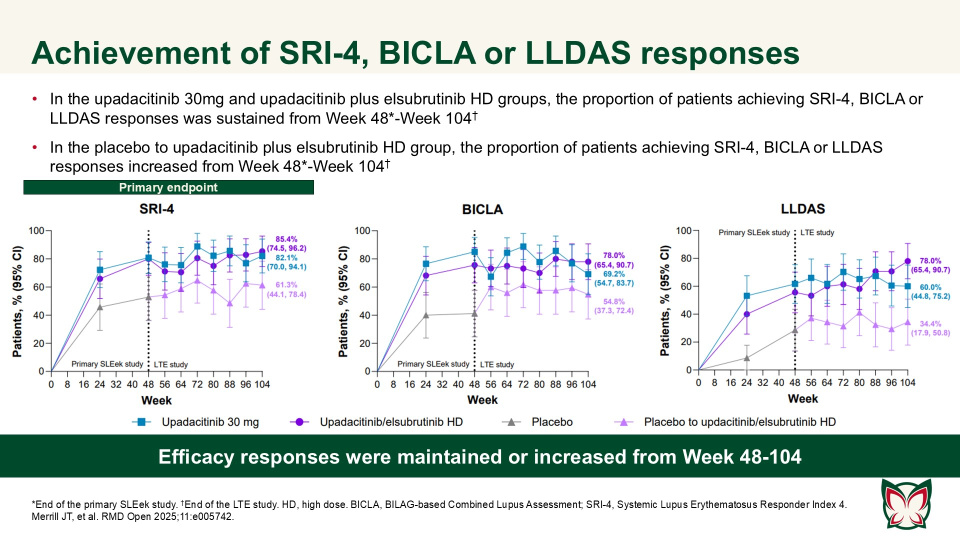
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
The long-term efficacy and safety of upadacitinib monotherapy or upadacitinib plus elsubrutinib combination therapy was evaluated. Eligible patients from the primary SLEek study continued upadacitinib monotherapy or combination therapy with elsubrutinib or were switched from placebo to combination therapy with elsubrutinib (R 1:1). The achievement of efficacy responses (Systemic Lupus Erythematosus Responder Index 4, BILAG-based Combined Lupus Assessment, LLDAS) were maintained or increased from baseline (Week 48) to Week 104. No new safety findings were observed for up to 104 weeks of continuous treatment.
Keywords: