Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
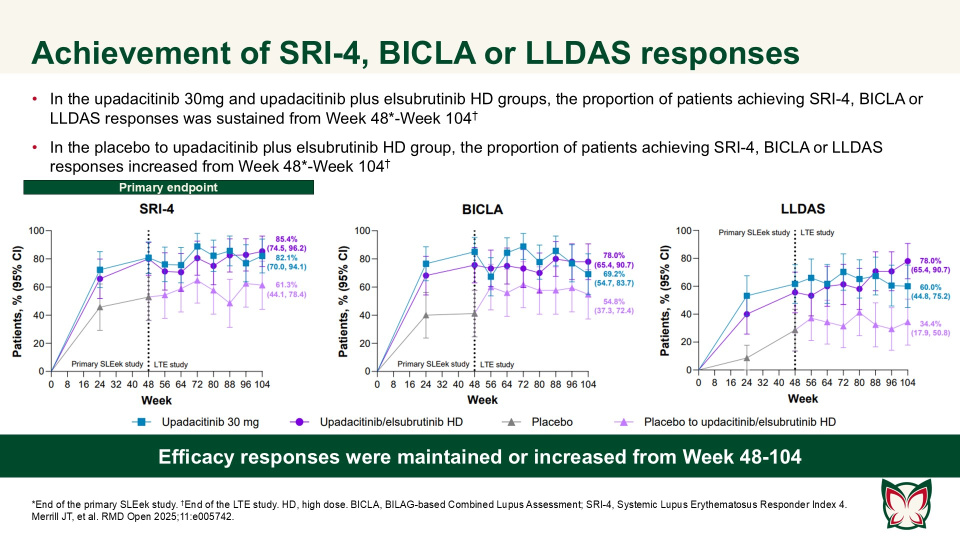
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials
Ann Rheum Dis. 2025:S0003-496700071-8. DOI: 10.1016/j.ard.2025.01.016. Epub ahead of print
Morand et al. conducted a post-hoc analysis of the phase III TULIP-1 and TULIP-2 trials and their long-term extension, including 369 patients with moderate to severe SLE, to evaluate the long-term impact of anifrolumab on attainment of LLDAS and DORIS-defined remission. The results demonstrated that anifrolumab significantly improved the likelihood, speed, and duration of LLDAS and DORIS remission versus placebo over 4 years, with benefits sustained throughout the treatment period.