Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
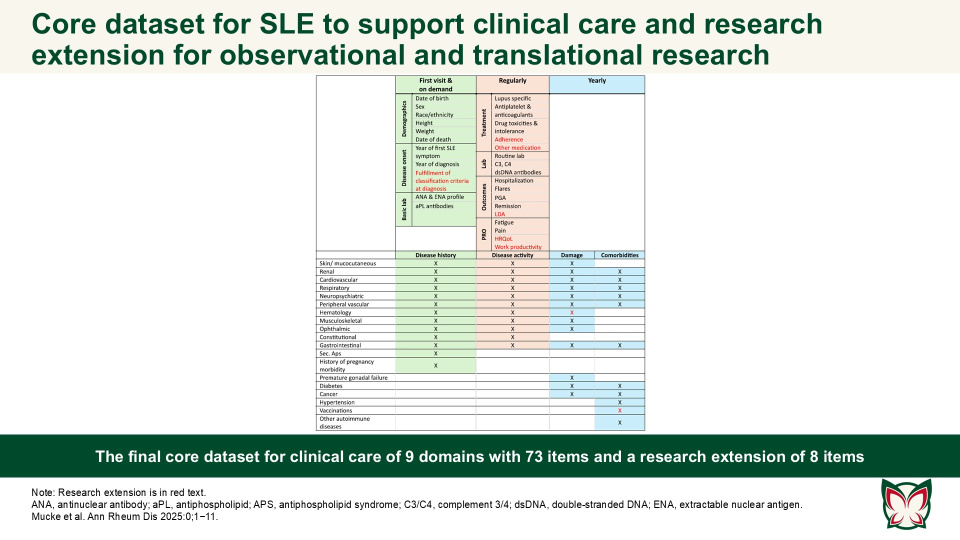
EULAR recommendations for a core dataset to support clinical care and translational and observational research in systemic lupus erythematosus
Ann Rheum Dis 2025:0;1−11 Doi: 10.1016/j.ard.2025.07.001
The presented clinical core dataset and its research extension are designed to improve SLE patient care and facilitate collaborative research by ensuring the comparability of datasets and cohort descriptions. The aim of this EULAR taskforce by Mucke et al. was to define a core set of essential items for the comprehensive care of SLE patients in clinical practice, with an extension for vital elements required for translational and observational research.
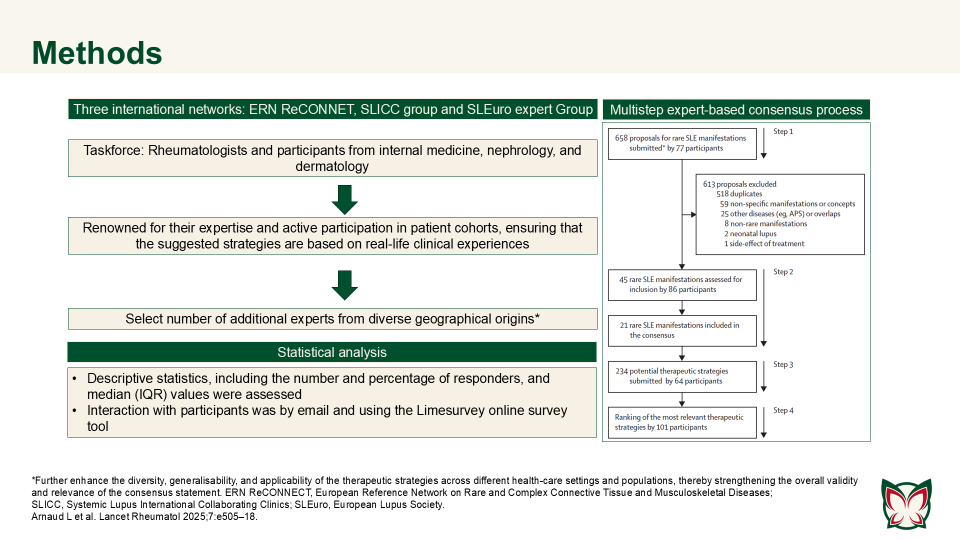
ERN ReCONNET–SLICC–SLEuro expert consensus on the therapeutic management of rare systemic lupus erythematosus manifestations
Lancet Rheumatol 2025;7:e505–18 Doi: 10.1016/S2665-9913(25)00063-3
This systematic review highlighted the major gaps in evidence regarding rare SLE manifestations, with many findings dependent on variable methodologies or single reports. Arnard et al. established an international consensus on therapeutic strategies for rare SLE manifestations.
Keywords:
Deucravacitinib, an oral, selective, allosteric, tyrosine kinase 2 inhibitor, in patients with active SLE: efficacy on patient-reported outcomes in a phase II randomised trial
Lupus Sci Med. 2025;12(1):e001517
Patient-reported outcomes from the deucravacitinib, 48-week, phase II, PAISLEY study show that patients with SLE experienced greater improvements in pain, fatigue and health-related quality-of-life scores at Week 48 with deucravacitinib versus placebo treatment.
Domains for inclusion in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE): results of a modified Delphi study
Lupus Sci Med. 2025 May 6;12(1):e001484 doi: 10.1136/lupus-2024-001484
Connelly, et al. use Delphi methods to achieve consensus to include eight domains of active disease to define treatment response in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE).
EULAR Recommendations for the Management of Systemic Lupus Erythematosus: 2023 Update
Ann Rheum Dis 2023;83(1):15–29 DOI: 10.1136/ard-2023-224762
The objective of this international task force was to update the EULAR recommendations for the management of SLE. The Task Force agreed on 5 overarching principles and 13 recommendations, generating an overall framework for the approach to a patient with SLE. The updated recommendations provide consensus guidance on the management of SLE, combining evidence and expert opinion.
Keywords:
Baricitinib for Systemic Lupus Erythematosus: a Double-blind, Randomised, Placebo-controlled, Phase 3 trial (SLE-BRAVE-II)
Lancet. 2023 doi: 10.1016/S0140-6736(22)02546-6
Negative results of SLE-BRAVE-II trial show that evidence for the efficacy of baricitinib in SLE is inconclusive.
Baricitinib for Systemic Lupus Erythematosus: a Double-blind, Randomised, Placebo-controlled, Phase 3 Trial (SLE-BRAVE-I)
Lancet. 2023 doi: 10.1016/S0140-6736(22)02607-1
Primary endpoint in SLE-BRAVE-I study was met for the 4 mg baricitinib group, however, key secondary endpoints were not.