Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
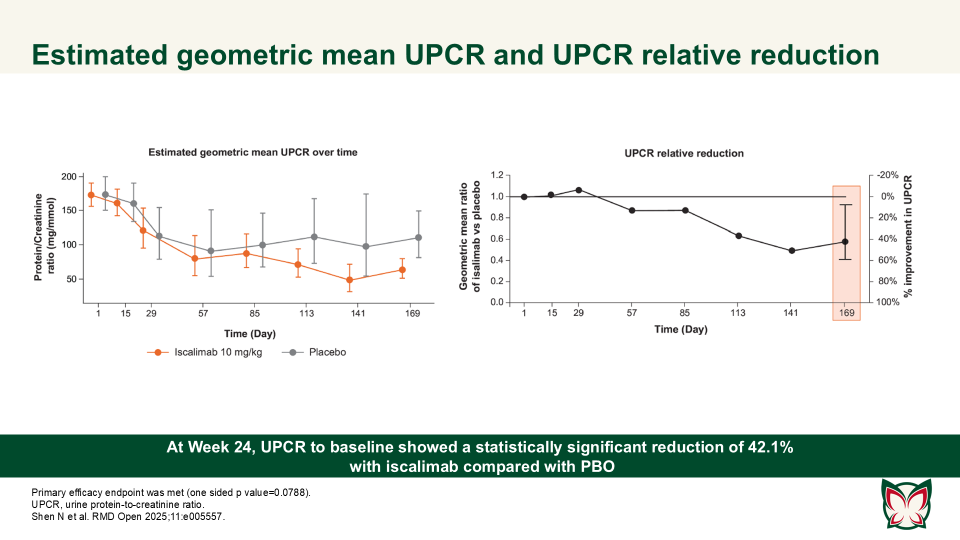
Efficacy, pharmacokinetics and safety of iscalimab (CFZ533) in patients with proliferative lupus nephritis: A randomised, double-blind, placebo-controlled, Phase II study
RMD Open 2025;11:e005557 Doi:10.1136/rmdopen-2025-005557
Shen N et al. report that iscalimab was clinically effective and generally well tolerated; in addition, it was devoid of the thromboembolic risk, characteristic of Fc active anti-CD40L therapies.
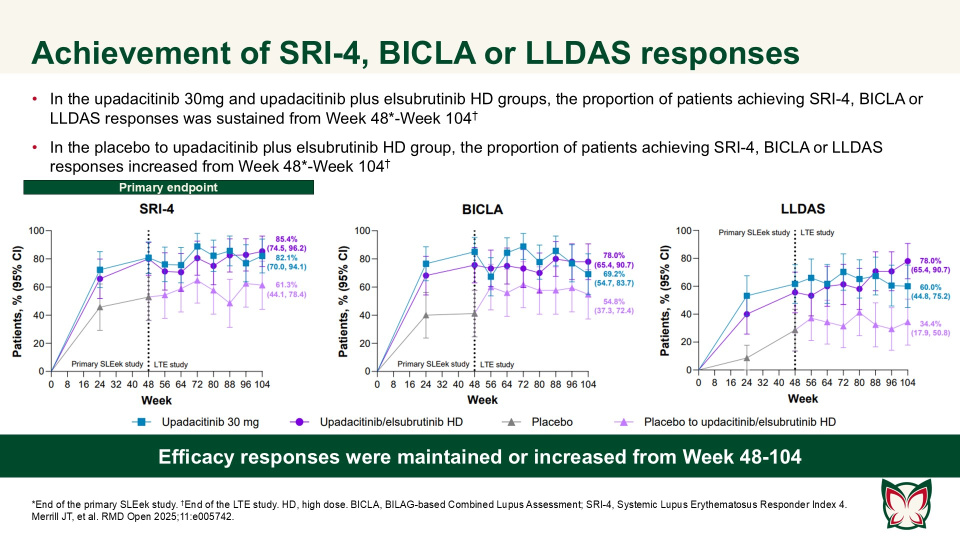
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
Cenerimod, a sphingosine-1-phosphate receptor modulator, versus placebo in patients with moderate-to-severe systemic lupus erythematosus (CARE): an international, double-blind, randomised, placebo-controlled, phase 2 trial
Lancet Rheumatol. 2024. Epub ahead of print. DOI: 10.1016/S2665-9913(24)00246-7
Askanase et al. assessed the efficacy, safety, and tolerability of cenerimod in patients with moderate-to-severe SLE. While the primary endpoint of reducing mSLEDAI-2K scores at Month 6 was not achieved, cenerimod 4.0mg showed a significant reduction in disease activity versus placebo. Adverse events, including lymphopenia, were dose-dependent but manageable, and overall treatment was well tolerated.
Comparison of a voclosporin-based triple immunosuppressive therapy to high-dose glucocorticoid-based immunosuppressive therapy: A propensity analysis of the AURA-LV and AURORA 1 studies and ALMS
Lupus Science & Medicine 2024;11:e001319 DOI: 10.1136/lupus-2024-001319
Dall’Era et al. conducted a propensity analysis to compare voclosporin-based triple immunosuppressive therapy with high-dose GC-based regimens for active LN. Voclosporin showed fewer AEs, improved safety, and significantly reduced proteinuria over six months, suggesting a superior risk-benefit profile for patients with lupus nephritis.
Keywords:
Effect of iberdomide on cutaneous manifestations in systemic lupus erythematosus: a randomized phase 2 clinical trial
JAAD. 2024. Epub ahead of print DOI: 10.1016/j.jaad.2024.09.074
Werth et al. demonstrated that iberdomide significantly improved cutaneous lupus erythematosus (CLE) outcomes, particularly in subacute and chronic CLE patients, by reducing Cutaneous Lupus Area and Severity Index Activity (CLASI-A) scores. The study showed continued efficacy through 24 weeks, with the 0.45 mg dose providing the greatest improvement in patients with severe baseline scores, and iberdomide was well-tolerated over 104 weeks.
Keywords:
Efficacy and safety of upadacitinib or elsubrutinib alone or in combination for systemic lupus erythematosus: A Phase 2 randomized controlled trial
Arthritis Rheumatol 2024 DOI: 10.1002/art.42926 Epub ahead of print
Daily oral upadacitinib 30 mg and ABBV-599 high dose (elsubrutinib 60 mg QD + upadacitinib 30 mg) were effective in multiple outcome measures including disease activity, flares, time to first flare, and joint counts.
Keywords:
Evaluation of RNase Therapy in Systemic Lupus Erythematosus: A Randomised Phase 2a Clinical Trial of RSLV-132
Lupus Sci Med. 2024;11:e001113 DOI 10.1136/lupus-2023-001113
Treatment with RSLV-132 was associated with lower rates of SAEs than placebo, although RSLV-132 therapy was not associated with a significant improvement in the mean CLASI score relative to placebo. However, results suggest that further evaluations of RSLV-132 in SLE should be undertaken with patients with more active disease who are most likely to benefit from RNase therapy.
Kidney Outcomes and Preservation of Kidney Function with Obinutuzumab in Patients with Lupus Nephritis: A Post Hoc Analysis of the NOBILITY Trial
Arthritis Rheumatol. 2023 DOI 10.1002/art.42734
This post hoc analysis of the Phase 2 NOBILITY trial determined that obinutuzumab plus standard of care improved the likelihood of long-term preservation of kidney function and improved kidney function with less glucocorticoid use in patients with lupus nephritis.
Keywords:
Telitacicept in Patients with Active Systemic Lupus Erythematosus: Results of A Phase 2b, Randomised, Double-blind, Placebo-controlled Trial
Ann Rheum Dis. 2023; DOI: 10.1136/ard-2023-224854
This Phase 2 trial demonstrated the efficacy and acceptable safety profile of telitacicept in patients with SLE. The safety profile of telitacicept was comparable with that observed in clinical trials of other B cell-targeting agents.
Keywords:
Emerging Immunotherapeutic Strategies for Cutaneous Lupus Erythematosus: An Overview of Recent Phase 2 and 3 Clinical Trials
Expert Opin Emerg Drugs 2023;28(4):257–73 DOI: 10.1080/14728214.2023.2273536
Current treatments for refractory CLE are insufficient, often leading to suboptimal disease control, and demanding escalated therapies associated with systemic toxicities. However, recent Phase 2 & 3 trials offer promising results, suggesting new therapeutics approved for CLE are on the horizon.