Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
Secukinumab in active lupus nephritis: Results from Phase III, randomised, placebo controlled study (SELUNE) and open-label extension study
Rheumatology 2025 Doi: 10.1093/rheumatology/keaf536 Epub ahead of print
Secukinumab did not demonstrate superior efficacy over PBO in patients with active LN; secukinumab was well-tolerated with no new or unexpected safety signals detected. A Phase-III core study (SELUNE) and an extension study, were conducted by Zhao et al. to evaluate the efficacy and safety of SC secukinumab 300mg compared with PBO, in combination with the SoC, in patients with active LN.
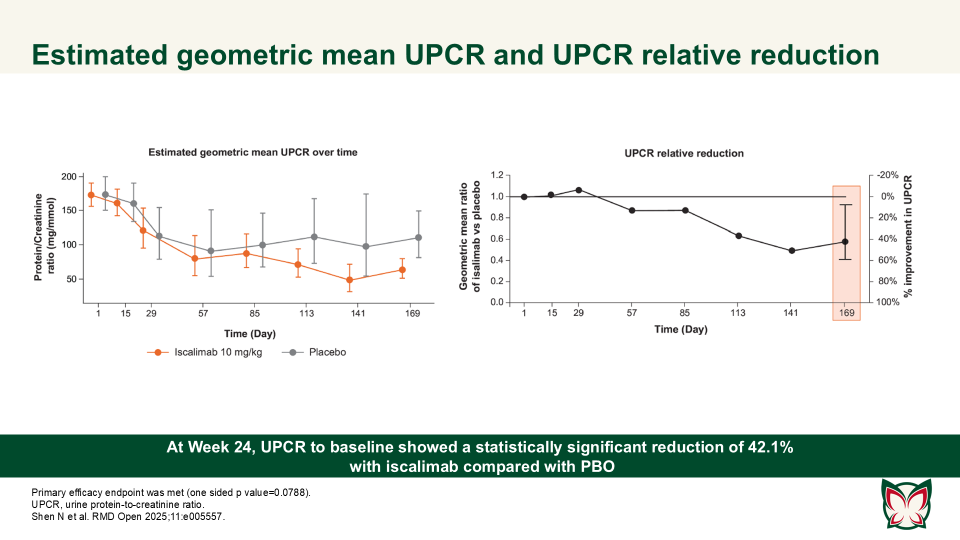
Efficacy, pharmacokinetics and safety of iscalimab (CFZ533) in patients with proliferative lupus nephritis: A randomised, double-blind, placebo-controlled, Phase II study
RMD Open 2025;11:e005557 Doi:10.1136/rmdopen-2025-005557
Shen N et al. report that iscalimab was clinically effective and generally well tolerated; in addition, it was devoid of the thromboembolic risk, characteristic of Fc active anti-CD40L therapies.
Deucravacitinib, an oral, selective, allosteric, tyrosine kinase 2 inhibitor, in patients with active SLE: efficacy on patient-reported outcomes in a phase II randomised trial
Lupus Sci Med. 2025;12(1):e001517
Patient-reported outcomes from the deucravacitinib, 48-week, phase II, PAISLEY study show that patients with SLE experienced greater improvements in pain, fatigue and health-related quality-of-life scores at Week 48 with deucravacitinib versus placebo treatment.
Outcomes of patients with systemic lupus erythematosus treated with belimumab: a post hoc efficacy analysis of five phase III clinical trials by British Isles Lupus Assessment Group-based Combined Lupus Assessment criteria
RMD Open 2025;11:e005-444 DOI:10.1136/rmdopen-2025-005444
Padrodis et al. aimed to determine belimumab efficacy assessed using BICLA in patients with SLE included in the phase III belimumab RCTs. The benefit of belimumab was found to be more prominent when combined with anti-malarial agents. Furthermore, using BICLA, the authors validated the results from foundational trials originally assessing belimumab efficacy using SLE Responder Index 4 thus, corroborating the efficacy of #belimumab in SLE.
Unsupervised machine learning identifies distinct systemic lupus erythematosus patient endotypes with differential response to belimumab
Rheumatol (Oxford), 2025 DOI: 10.1093/rheumatology/keaf215. Epub ahead of print
Depascale et al. used unsupervised machine learning to identify three SLE endotypes based on B cell immunophenotyping and serological profiles. Belimumab was most effective in patients with transitional and naïve B cell enrichment (Cluster 2), where it significantly increased the likelihood of achieving sustained LLDAS and DORIS remission.
Keywords:
LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials
Ann Rheum Dis. 2025:S0003-496700071-8. DOI: 10.1016/j.ard.2025.01.016. Epub ahead of print
Morand et al. conducted a post-hoc analysis of the phase III TULIP-1 and TULIP-2 trials and their long-term extension, including 369 patients with moderate to severe SLE, to evaluate the long-term impact of anifrolumab on attainment of LLDAS and DORIS-defined remission. The results demonstrated that anifrolumab significantly improved the likelihood, speed, and duration of LLDAS and DORIS remission versus placebo over 4 years, with benefits sustained throughout the treatment period.
Keywords:
Belimumab efficacy in mucocutaneous lupus erythematosus: a large post-hoc analysis from five phase III clinical trials
Rheumatol, 2025. Epub ahead of print. DOI: 10.1093/rheumatology/keaf145
Grosso et al. conducted a post-hoc analysis of five phase III trials, including 3086 patients, to evaluate the efficacy of belimumab in improving mucocutaneous manifestations of SLE. The results demonstrated that belimumab significantly improved disease activity as measured by the mcBILAG and mcSLEDAI-2K indices compared with placebo and reduced flare rates in patients with high disease activity and serological positivity.
Keywords:
Predictors of Renal Flares in Systemic Lupus Erythematosus: A Post-hoc Analysis of Four Phase III Clinical Trials of Belimumab
Rheumatology (Oxford) 2024 DOI: 10.1093/rheumatology/keae023 Epub ahead of print
High baseline proteinuria levels, hypoalbuminaemia, and C3 consumption were associated with
renal flare development. Renal flares remain common in patients with SLE, however causative factors are still largely unknown. Jagerback, et al. conducted a post-hoc analysis of pooled BLISS trial data to identify predictors of renal flares.
Keywords:
Update on the Efficacy and Safety Profile of Voclosporin: An Integrated Analysis of Clinical Trials in Lupus Nephritis
Arthritis Care Res (Hoboken). 2023 Jul;75(7):1399–1408 DOI: 10.1002/acr.25007
Pooled analysis of data from the AURA-LV phase 2 and AURORA 1 phase 3 trials of voclosporin in patients with active LN demonstrated that significantly more patients achieved a complete renal response at 1 year in the voclosporin than the control group (p<0.0001), with no observation of new safety signals.
Belimumab and antimalarials combined against renal flares in patients treated for extra-renal systemic lupus erythematosus: results from 4 phase III clinical trials
Rheumatology (Oxford) 2023;63(2):338–48 doi: 10.1093/rheumatology/kead253
The protection conferred from belimumab against renal flare development in patients treated for extra-renal SLE appears enhanced when administered along with antimalarials (AMA).