Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
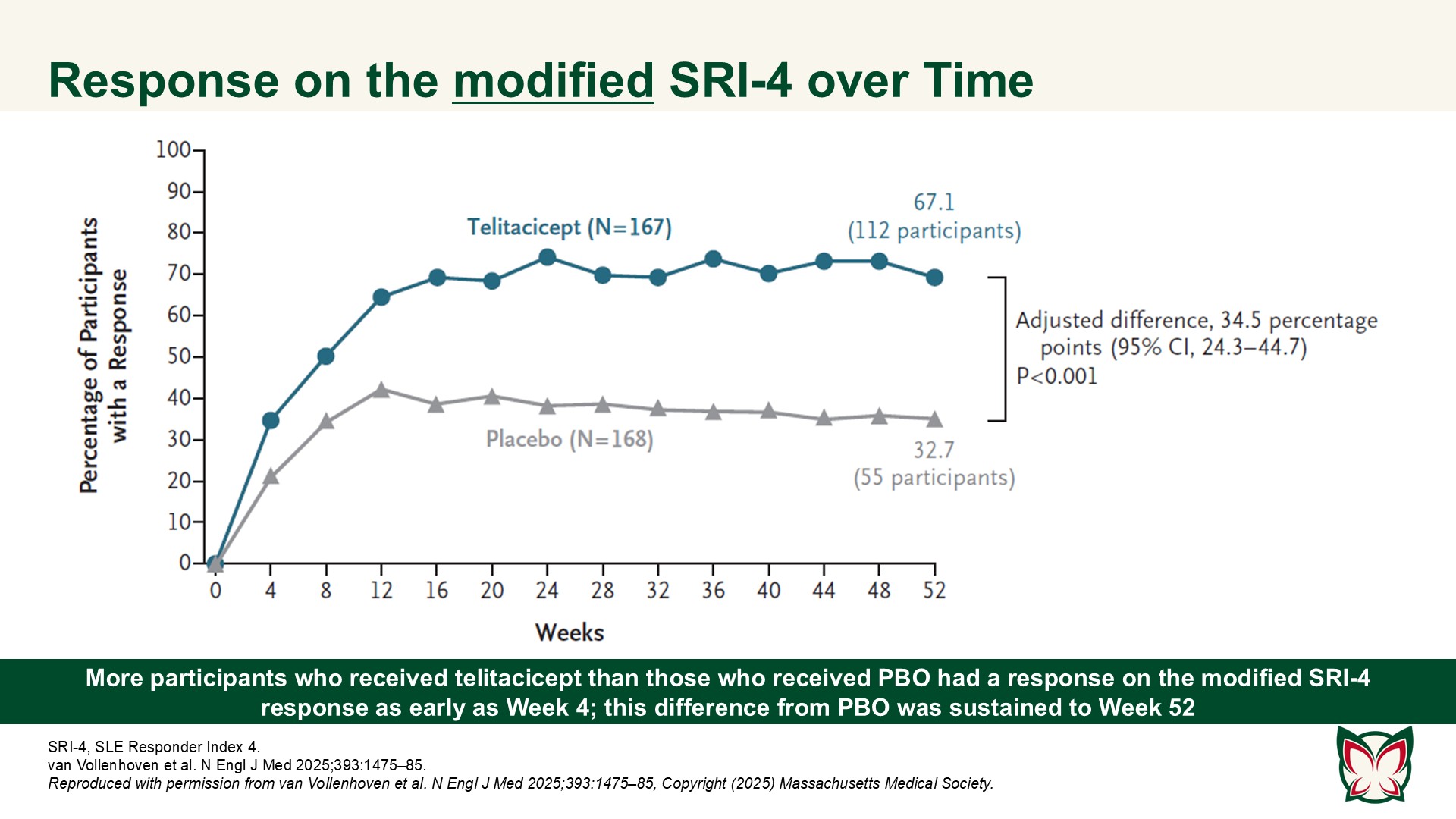
A Phase 3 trial of telitacicept for systemic lupus erythematosus
N Engl J Med 2025;393:1475-85 Doi: 10.1056/NEJMoa2414719
In this 52-week trial involving participants with active SLE who were receiving background therapy, the incidence of a clinical response was higher with telitacicept than with PBO. van Vollenhoven et al. report efficacy and safety results of a Phase 3 trial of telitacicept at a dose of 160mg weekly as compared with PBO in Chinese persons with active SLE.
Safety, pharmacokinetics, biomarker response and efficacy of E6742: a dual antagonist of Toll-like receptors 7 and 8, in a first-in-patient, randomised, double-blind, phase I/II study in systemic lupus erythematosus
RMD Open 2024;10:e004701 DOI 10.1136/rmdopen-2024-004701
Tanaka et al. conducted a phase I/II study to evaluate the safety, pharmacokinetics, biomarker response, and efficacy of E6742, a dual antagonist of Toll-like receptors 7 and 8, in patients with systemic lupus erythematosus (SLE). The treatment demonstrated a favourable safety profile, with no serious adverse events, while effectively suppressing interferon gene signatures and showing promising preliminary efficacy.