Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
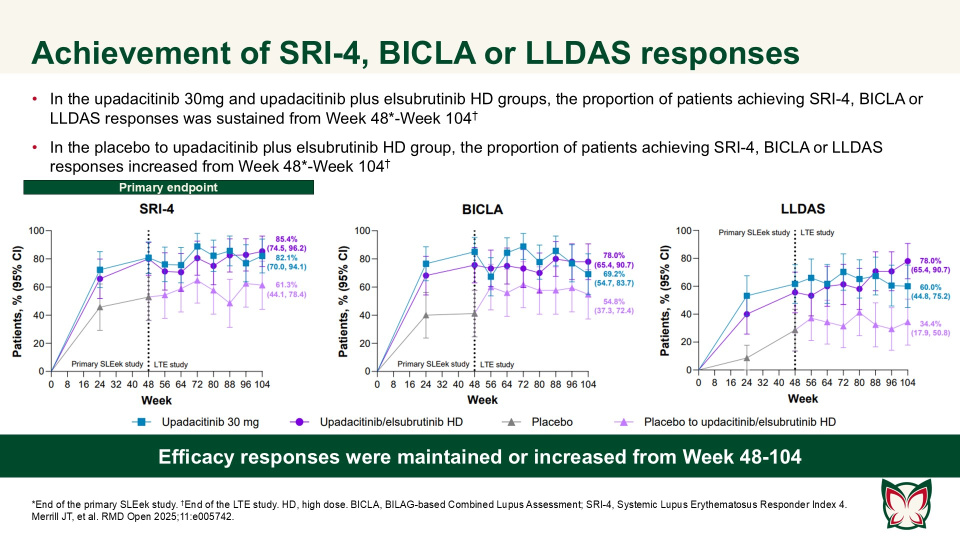
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
Domains for inclusion in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE): results of a modified Delphi study
Lupus Sci Med. 2025 May 6;12(1):e001484 doi: 10.1136/lupus-2024-001484
Connelly, et al. use Delphi methods to achieve consensus to include eight domains of active disease to define treatment response in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE).
Outcomes of patients with systemic lupus erythematosus treated with belimumab: a post hoc efficacy analysis of five phase III clinical trials by British Isles Lupus Assessment Group-based Combined Lupus Assessment criteria
RMD Open 2025;11:e005-444 DOI:10.1136/rmdopen-2025-005444
Padrodis et al. aimed to determine belimumab efficacy assessed using BICLA in patients with SLE included in the phase III belimumab RCTs. The benefit of belimumab was found to be more prominent when combined with anti-malarial agents. Furthermore, using BICLA, the authors validated the results from foundational trials originally assessing belimumab efficacy using SLE Responder Index 4 thus, corroborating the efficacy of #belimumab in SLE.
Assessment and Personalised Advice for Fatigue in Systemic Lupus Erythematosus Using an Innovative Digital Tool: The Lupus Expert System for the Assessment of Fatigue (LEAF) Study
RMD open. 2023; DOI:10.1136/rmdopen-2023-003476
Kawka et al investigated the use of the LEAF digital health tool to successfully assess the extent of patient-reported fatigue in SLE patients.
Risk and Factors Associated with Disease Manifestations in Systemic Lupus Erythematosus - Lupus Nephritis (RIFLE-LN): A Ten-year Risk Prediction Strategy Derived from a Cohort of 1652 Patients
Front Immunol. 2023 14:1200732 DOI: 10.3389/fimmu.2023.1200732
Using data from a longitudinal cohort of SLE patients of Chinese origin, the authors identified risk factors for LN and developed a prediction model, RIFLE-LN, which demonstrated good performance in a testing cohort of 270 patients.
Early Infection Risk in Patients with Systemic Lupus Erythematosus Treated with Rituximab or Belimumab from the British Isles Lupus Assessment Group Biologics Register (BILAG-BR): A Prospective Longitudinal Study
Lancet Rheumatol 2023;5:e284–92 doi:https://doi.org/10.1016/S2665-9913(23)00091-7
Data from a large prospective registry (BILAG-BR) highlight that, compared with standard of care, the serious infection risk was similar between rituximab and belimumab.
Keywords:
Does Remission in Systemic Lupus Erythematosus According to the 2021 DORIS Definition Match the Treating Rheumatologist’s Judgment?
Rheumatology (Oxford) 2023;63(1):72–8 doi: 10.1093/rheumatology/kead159
Assessment on agreement between the 2021 DORIS and physician-judged lupus activity finds the 2021 DORIS remission to be an achievable target in clinical practice, with substantial agreement between the DORIS definition and physician-judged remission.
Keywords:
Evaluation of the LFA-REAL Clinician-reported Outcome (ClinRO) and Patient-reported Outcome (PRO): Prespecified Analysis of the Phase III Ustekinumab Trial in Patients with SLE
Lupus Sci Med. 2023 doi:10.1136/lupus-2022-000875
The Lupus Foundation of America Rapid Evaluation of Activity in Lupus (LFA-REAL) clinician-reported outcome (ClinRO) and patient-reported outcome (PRO) systems show potential as a flexible resource in the evaluation of lupus disease activity and a simple, user-friendly outcome measure for SLE studies.
Keywords:
Clinical meaningfulness of a British Isles Lupus Assessment Group-based Composite Lupus Assessment response in terms of patient-reported outcomes in moderate to severe systemic lupus erythematosus: a post-hoc analysis of the phase 3 TULIP-1 and TULIP-2 trials of anifrolumab
Lancet Rheumatol 2022;4:e198–207
In patients with moderate-to-severe SLE, British Isles Lupus Assessment Group-based Composite Lupus Assessment (BICLA) responders report improvements in disease activity, health-related quality of life, fatigue, and pain.
Easy-BILAG: a new tool for simplified recording of SLE disease activity using BILAG-2004 index
Rheumatology (Oxford). 2022. Epub ahead of print doi: 10.1093/rheumatology/keab883
Easy-BILAG is a high-accuracy, time-efficient tool for recording BILAG-2004 disease activity in systemic lupus erythematosus (SLE).
Disease activity measurements in SLE are necessary for optimal patient care, treat-to-target approaches and clinical guidelines. However, administrative burden and potential frequency of errors with the current comprehensive disease activity instrument (BILAG-2004) limits its use in routine practice.