Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
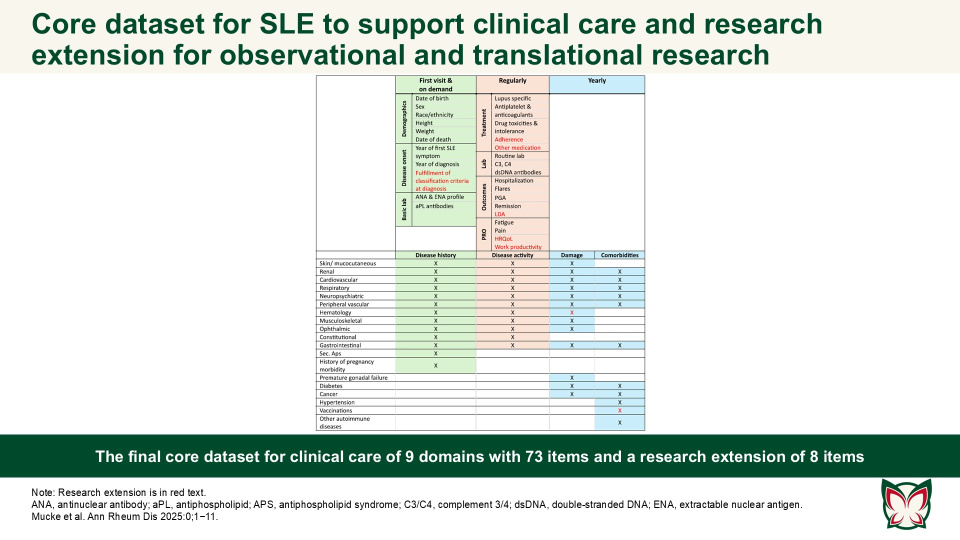
EULAR recommendations for a core dataset to support clinical care and translational and observational research in systemic lupus erythematosus
Ann Rheum Dis 2025:0;1−11 Doi: 10.1016/j.ard.2025.07.001
The presented clinical core dataset and its research extension are designed to improve SLE patient care and facilitate collaborative research by ensuring the comparability of datasets and cohort descriptions. The aim of this EULAR taskforce by Mucke et al. was to define a core set of essential items for the comprehensive care of SLE patients in clinical practice, with an extension for vital elements required for translational and observational research.
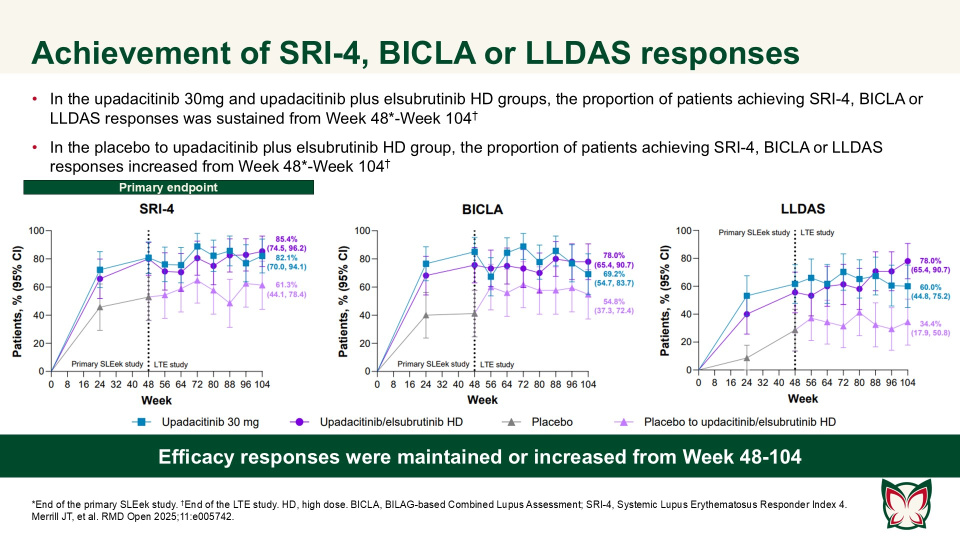
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
Type I interferon blockade with anifrolumab in patients with systemic lupus erythematosus modulates key immunopathological pathways in a gene expression and proteomic analysis of two Phase 3 trials
Ann Rheum Dis 2024 DOI 10.1136/ard-2023-225445 Epub ahead of print https://pubmed.ncbi.nlm.nih.gov/38569851/
Type I IFN blockade with anifrolumab modulated multiple inflammatory pathways downstream of type I IFN signalling.
TYK2: An emerging therapeutic target in rheumatic disease
Nat Rev Rheumatol 2024;20(4):232–40 DOI 10.1038/s41584-024-01093-w
TYK2 inhibitors hold promise for the treatment of a distinct spectrum of autoimmune diseases, including SLE, and could potentially have a safety profile that differs from other JAK inhibitors.