Anti-CD38 monoclonal antibody CM313 for systemic lupus erythematosus: A randomized, double-blind, placebo-controlled Phase Ib/IIa trial
Signal Transduct Target Ther 2025;10:383 Doi: 10.1038/s41392-025-02487-2
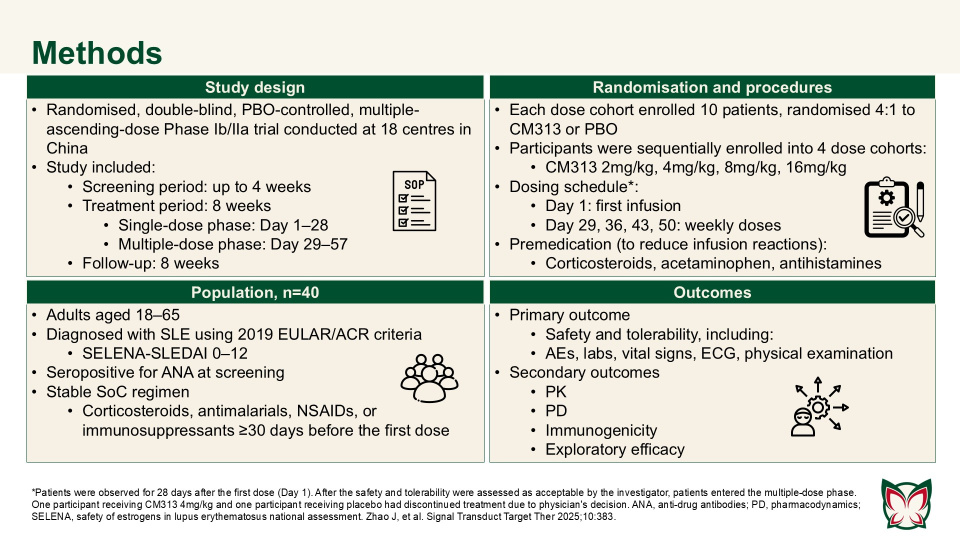
Zhao et al. showed that CM313 was well tolerated in adult patients with SLE at doses of 2–16mg/kg and showed encouraging pharmacodynamic effects and preliminary efficacy at doses of 8 and 16 mg/kg QW. CM313 also produced dose-dependent and clinically meaningful improvements in key serological biomarkers of SLE.
The results of this study support further investigation of CM313 as a new treatment option for patients with SLE.