Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
Efficacy and safety of allogeneic CD19 CAR NK-cell therapy in systemic lupus erythematosus: A case series in China
Lancet 2025 Doi:10.1016/S0140-6736(25)01671-X Epub ahead of print
Gao et al. report that allogeneic CAR NK-cell therapy is a potent option for treatment of autoimmune diseases and may address limitations of current autologous CAR T-cell therapy, including manufacturing scale and time, access, safety, and cost. Authors evaluated the safety, tolerability, and efficacy of allogeneic CD19 CAR NK-cell therapy in patients with relapsed or refractory SLE.
Keywords:
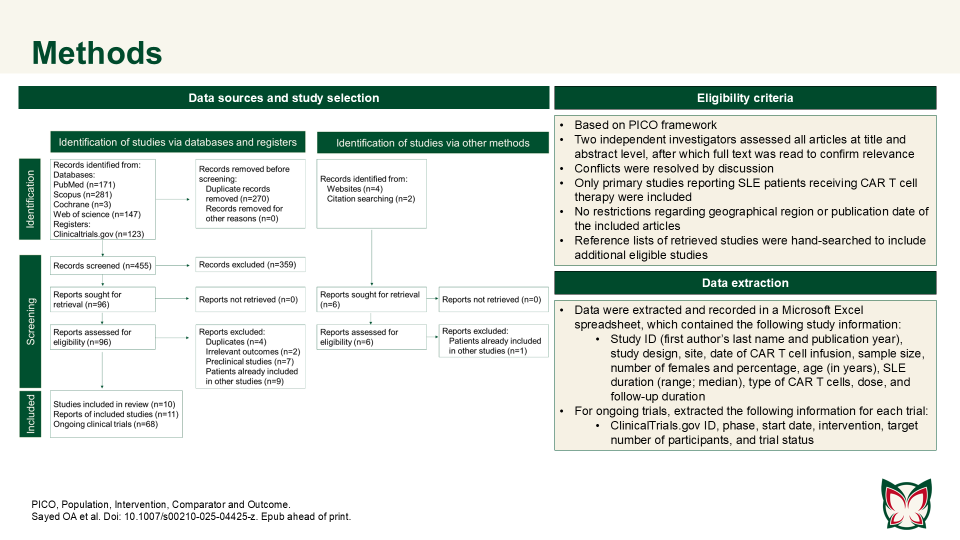
CAR T cell therapy efficacy and safety in SLE: A systematic review and pooled analysis of 47 patients across 10 studies
Doi: 10.1007/s00210-025-04425-z
Sayed OA et al. reported that CAR T cell therapy showed promise in refractory SLE, achieving durable remission with manageable toxicity; however further trials are needed to confirm long-term outcomes. Authors consolidated the current evidence on CAR T cell therapy in the treatment of SLE.
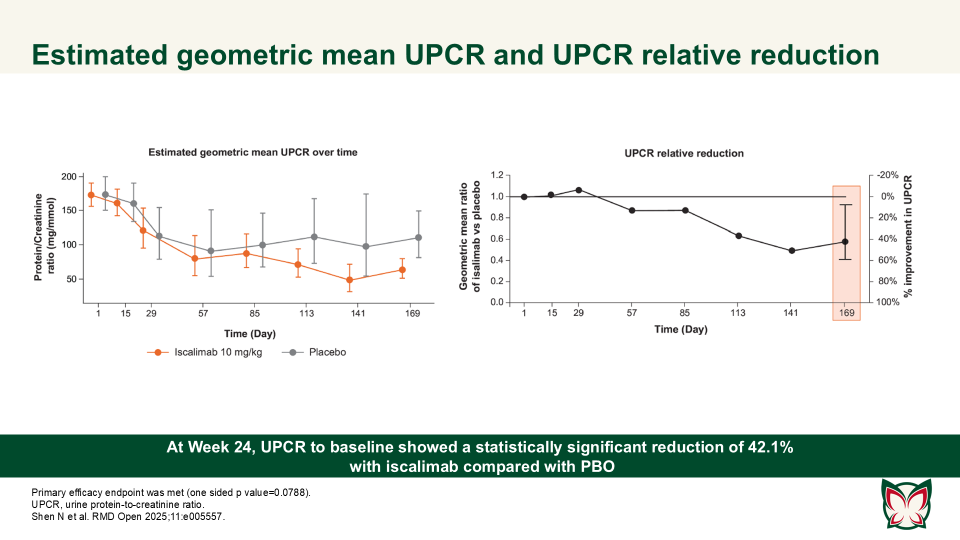
Efficacy, pharmacokinetics and safety of iscalimab (CFZ533) in patients with proliferative lupus nephritis: A randomised, double-blind, placebo-controlled, Phase II study
RMD Open 2025;11:e005557 Doi:10.1136/rmdopen-2025-005557
Shen N et al. report that iscalimab was clinically effective and generally well tolerated; in addition, it was devoid of the thromboembolic risk, characteristic of Fc active anti-CD40L therapies.
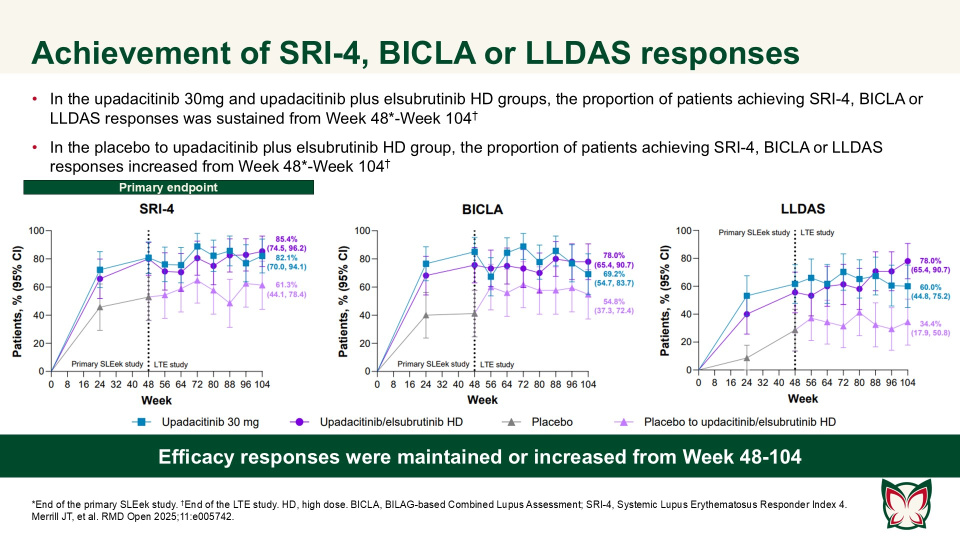
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
Effect of long-term voclosporin treatment on renal histology in patients with active lupus nephritis with repeat renal biopsies
Arthritis & Rheumatology 2025; 0:1–7 doi: 10.1002/art.43209
Exposure to voclosporin for a median of 18 months was not associated with onset or progression of nephrotoxicity based on evaluation of histologic compartments and vascular lesions. Rovin et al. characterised the impact of voclosporin on kidney histology in patients with LN who had protocolized repeat kidney biopsies in the AURORA clinical trials.
Keywords:
Deucravacitinib, an oral, selective, allosteric, tyrosine kinase 2 inhibitor, in patients with active SLE: efficacy on patient-reported outcomes in a phase II randomised trial
Lupus Sci Med. 2025;12(1):e001517
Patient-reported outcomes from the deucravacitinib, 48-week, phase II, PAISLEY study show that patients with SLE experienced greater improvements in pain, fatigue and health-related quality-of-life scores at Week 48 with deucravacitinib versus placebo treatment.
Domains for inclusion in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE): results of a modified Delphi study
Lupus Sci Med. 2025 May 6;12(1):e001484 doi: 10.1136/lupus-2024-001484
Connelly, et al. use Delphi methods to achieve consensus to include eight domains of active disease to define treatment response in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE).
Enhancing systemic lupus erythematosus treatment outcomes with an early initiation of belimumab: insights from a multicenter retrospective study within the first five years
Arthritis Res Ther 2025;27(1):116
Highlighting the importance of early belimumab initiation in SLE, patients with shorter disease duration achieve more substantial improvements in disease activity with early belimumab treatment.
Keywords:
2025 American College of Rheumatology (ACR) Guideline for the Treatment of Systemic Lupus Erythematosus (SLE) Guideline Summary
https://rheumatology.org/lupus-guideline#2025-sle-guideline
The ACR have published their summary of the 2025 Systemic Lupus Erythematosus Guideline. This updated lupus guideline project is divided into two manuscripts that include renal published (link) and non renal recommendations that are available online in summary now, and the manuscript format due to follow in late 2025.
Keywords:
Unsupervised machine learning identifies distinct systemic lupus erythematosus patient endotypes with differential response to belimumab
Rheumatol (Oxford), 2025 DOI: 10.1093/rheumatology/keaf215. Epub ahead of print
Depascale et al. used unsupervised machine learning to identify three SLE endotypes based on B cell immunophenotyping and serological profiles. Belimumab was most effective in patients with transitional and naïve B cell enrichment (Cluster 2), where it significantly increased the likelihood of achieving sustained LLDAS and DORIS remission.