Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
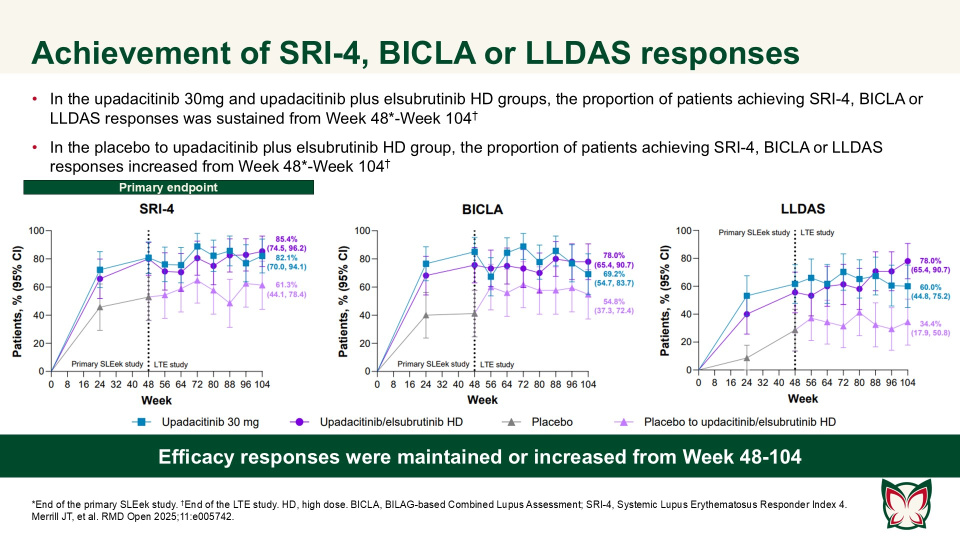
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
BCMA-targeted CAR T cell therapy can effectively induce disease remission in refractory lupus nephritis
Ann Rheum Dis 2025;0:1−9
Hu et al. report that anti-BCMA only CAR T cell can help LN patients safely and effectively, indicating its potential to be a feasible therapeutic strategy in treating autoimmune diseases with abnormal humoral immune responses.
Effect of long-term voclosporin treatment on renal histology in patients with active lupus nephritis with repeat renal biopsies
Arthritis & Rheumatology 2025; 0:1–7 doi: 10.1002/art.43209
Exposure to voclosporin for a median of 18 months was not associated with onset or progression of nephrotoxicity based on evaluation of histologic compartments and vascular lesions. Rovin et al. characterised the impact of voclosporin on kidney histology in patients with LN who had protocolized repeat kidney biopsies in the AURORA clinical trials.
Keywords:
Deucravacitinib, an oral, selective, allosteric, tyrosine kinase 2 inhibitor, in patients with active SLE: efficacy on patient-reported outcomes in a phase II randomised trial
Lupus Sci Med. 2025;12(1):e001517
Patient-reported outcomes from the deucravacitinib, 48-week, phase II, PAISLEY study show that patients with SLE experienced greater improvements in pain, fatigue and health-related quality-of-life scores at Week 48 with deucravacitinib versus placebo treatment.
Outcomes of patients with systemic lupus erythematosus treated with belimumab: a post hoc efficacy analysis of five phase III clinical trials by British Isles Lupus Assessment Group-based Combined Lupus Assessment criteria
RMD Open 2025;11:e005-444 DOI:10.1136/rmdopen-2025-005444
Padrodis et al. aimed to determine belimumab efficacy assessed using BICLA in patients with SLE included in the phase III belimumab RCTs. The benefit of belimumab was found to be more prominent when combined with anti-malarial agents. Furthermore, using BICLA, the authors validated the results from foundational trials originally assessing belimumab efficacy using SLE Responder Index 4 thus, corroborating the efficacy of #belimumab in SLE.
Long-term effect of anifrolumab on patient-reported outcomes in systemic lupus erythematosus (TULIP-LTE): a randomised, placebo-controlled, phase 3 long-term extension trial
Lancet Rheumatol, 2025 DOI 10.1016/S2665-9913(25)00022-0
Strand et al. performed an exploratory analysis to assess patient-reported outcome measures, to investigate how patients with moderate-to-severe SLE perceive the effects of long-term treatment with #anifrolumab on their health status and health-related quality of life. They report improvements in health status and health-related quality of life, including differences favouring anifrolumab compared with placebo. These numerical improvements in patient- reported outcomes occurred alongside improvements in disease activity, reduced glucocorticoid doses, and a tolerable safety profile. These data suggest that anifrolumab is an effective treatment option that might improve health-related quality of life.
Domains for inclusion in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE): results of a modified Delphi study
Lupus Sci Med. 2025 May 6;12(1):e001484 doi: 10.1136/lupus-2024-001484
Connelly, et al. use Delphi methods to achieve consensus to include eight domains of active disease to define treatment response in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE).
Enhancing systemic lupus erythematosus treatment outcomes with an early initiation of belimumab: insights from a multicenter retrospective study within the first five years
Arthritis Res Ther 2025;27(1):116
Highlighting the importance of early belimumab initiation in SLE, patients with shorter disease duration achieve more substantial improvements in disease activity with early belimumab treatment.
Keywords:
2025 American College of Rheumatology (ACR) Guideline for the Treatment of Systemic Lupus Erythematosus (SLE) Guideline Summary
https://rheumatology.org/lupus-guideline#2025-sle-guideline
The ACR have published their summary of the 2025 Systemic Lupus Erythematosus Guideline. This updated lupus guideline project is divided into two manuscripts that include renal published (link) and non renal recommendations that are available online in summary now, and the manuscript format due to follow in late 2025.
Keywords:
Unsupervised machine learning identifies distinct systemic lupus erythematosus patient endotypes with differential response to belimumab
Rheumatol (Oxford), 2025 DOI: 10.1093/rheumatology/keaf215. Epub ahead of print
Depascale et al. used unsupervised machine learning to identify three SLE endotypes based on B cell immunophenotyping and serological profiles. Belimumab was most effective in patients with transitional and naïve B cell enrichment (Cluster 2), where it significantly increased the likelihood of achieving sustained LLDAS and DORIS remission.