Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
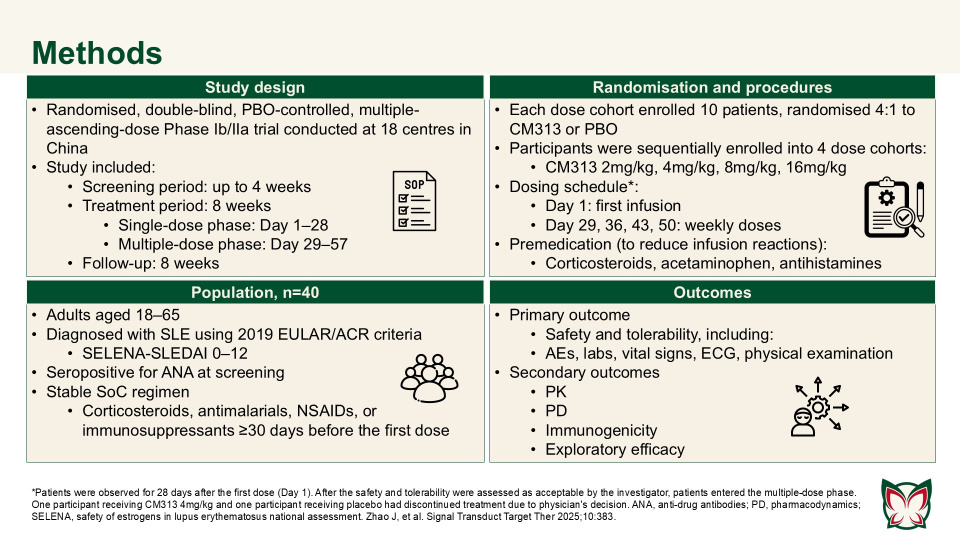
Anti-CD38 monoclonal antibody CM313 for systemic lupus erythematosus: A randomized, double-blind, placebo-controlled Phase Ib/IIa trial
Signal Transduct Target Ther 2025;10:383 Doi: 10.1038/s41392-025-02487-2
Zhao et al. showed that CM313 was well tolerated in adult patients with SLE at doses of 2–16mg/kg and showed encouraging pharmacodynamic effects and preliminary efficacy at doses of 8 and 16 mg/kg QW. CM313 also produced dose-dependent and clinically meaningful improvements in key serological biomarkers of SLE.
Efficacy and safety of allogeneic CD19 CAR NK-cell therapy in systemic lupus erythematosus: A case series in China
Lancet 2025 Doi:10.1016/S0140-6736(25)01671-X Epub ahead of print
Gao et al. report that allogeneic CAR NK-cell therapy is a potent option for treatment of autoimmune diseases and may address limitations of current autologous CAR T-cell therapy, including manufacturing scale and time, access, safety, and cost. Authors evaluated the safety, tolerability, and efficacy of allogeneic CD19 CAR NK-cell therapy in patients with relapsed or refractory SLE.
Keywords:
Unsupervised machine learning identifies distinct systemic lupus erythematosus patient endotypes with differential response to belimumab
Rheumatol (Oxford), 2025 DOI: 10.1093/rheumatology/keaf215. Epub ahead of print
Depascale et al. used unsupervised machine learning to identify three SLE endotypes based on B cell immunophenotyping and serological profiles. Belimumab was most effective in patients with transitional and naïve B cell enrichment (Cluster 2), where it significantly increased the likelihood of achieving sustained LLDAS and DORIS remission.
Keywords:
Opportunities and limitations of B bell depletion approaches in SLE
Nature Review Rheumatol, 2025;21:111–126 DOI: 10.1038/s41584-024-01210-9
Stockfelt et al. reviewed the long-term efficacy and challenges of B cell depletion strategies in SLE. Rituximab, a CD20-targeting monoclonal antibody, has demonstrated efficacy in a subset of patients but remains limited by immunogenicity, residual B cells, and B-cell activating factor (BAFF)-mediated relapse. Newer strategies incorporating CAR T cells, bispecific T cell engagers, and combination therapies aim to enhance B cell depletion and optimise outcomes.
Keywords:
Efficacy and safety of obinutuzumab in active lupus nephritis
NEJM, 2025. Epub ahead of print. DOI: 10.1056/NEJMoa2410965
Furie et al. demonstrated that obinutuzumab plus standard therapy significantly improved complete renal response at Wk76 compared with placebo. No unexpected safety signals were identified, though infections and COVID-19-related events were more frequent in the obinutuzumab group.