Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
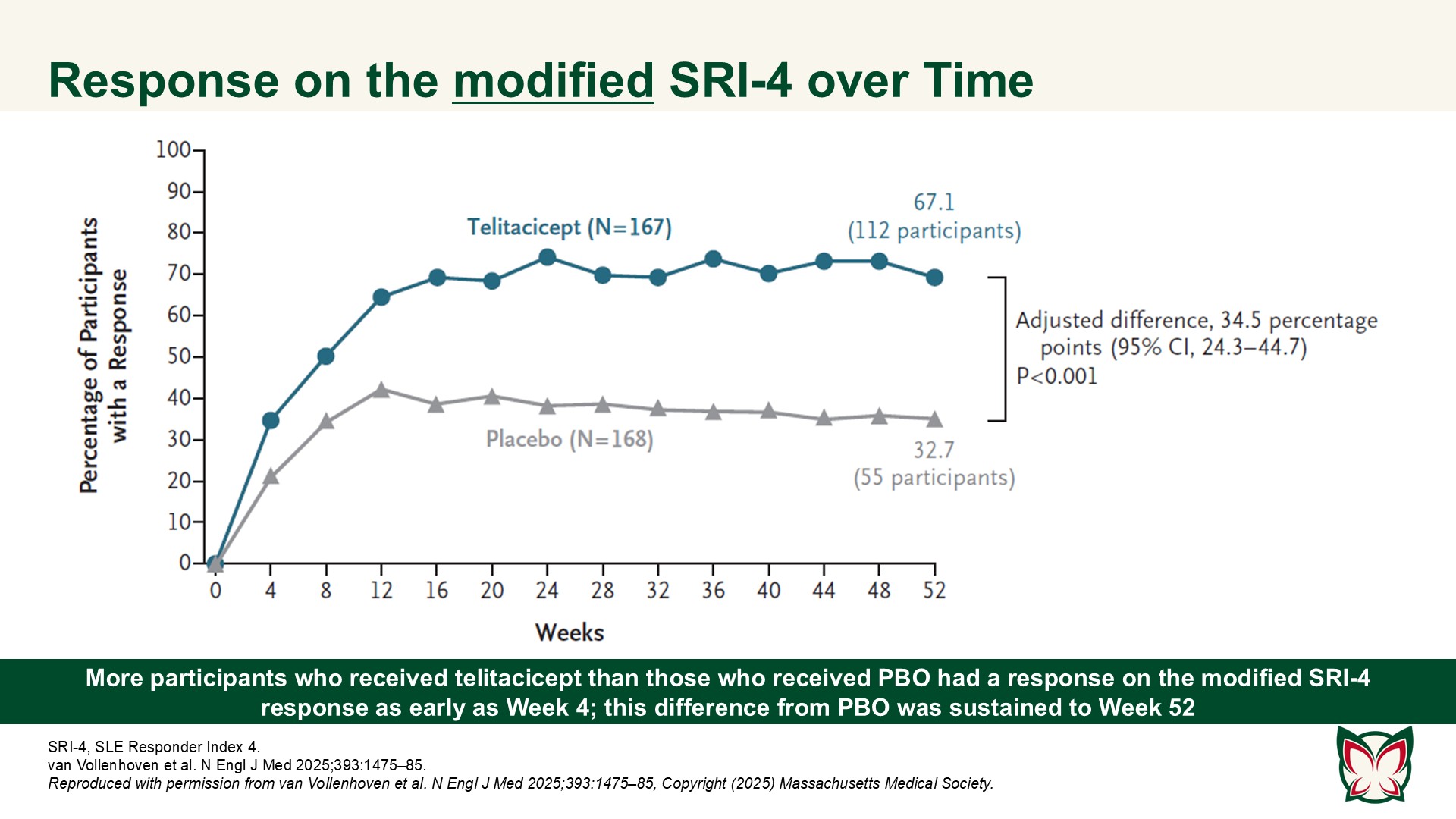
A Phase 3 trial of telitacicept for systemic lupus erythematosus
N Engl J Med 2025;393:1475-85 Doi: 10.1056/NEJMoa2414719
In this 52-week trial involving participants with active SLE who were receiving background therapy, the incidence of a clinical response was higher with telitacicept than with PBO. van Vollenhoven et al. report efficacy and safety results of a Phase 3 trial of telitacicept at a dose of 160mg weekly as compared with PBO in Chinese persons with active SLE.
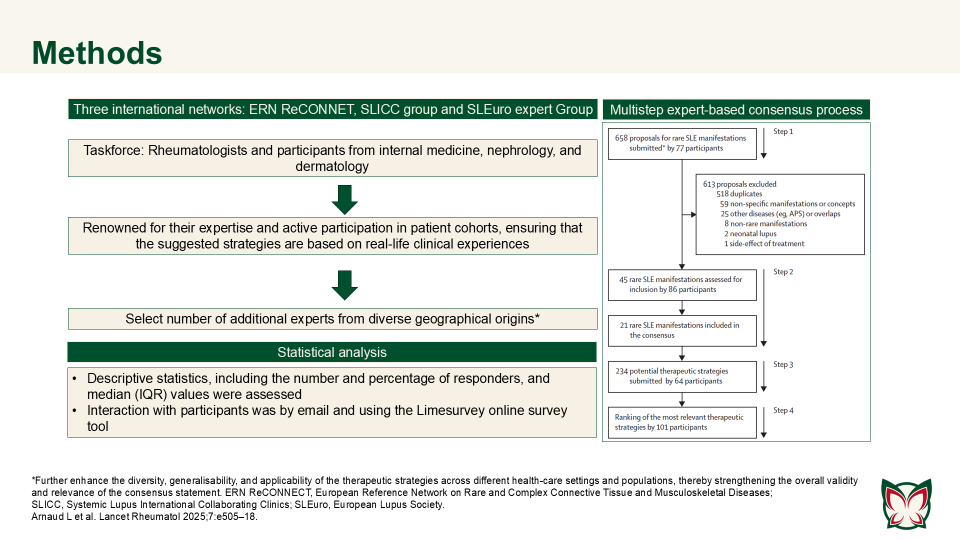
ERN ReCONNET–SLICC–SLEuro expert consensus on the therapeutic management of rare systemic lupus erythematosus manifestations
Lancet Rheumatol 2025;7:e505–18 Doi: 10.1016/S2665-9913(25)00063-3
This systematic review highlighted the major gaps in evidence regarding rare SLE manifestations, with many findings dependent on variable methodologies or single reports. Arnard et al. established an international consensus on therapeutic strategies for rare SLE manifestations.
Keywords:
Domains for inclusion in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE): results of a modified Delphi study
Lupus Sci Med. 2025 May 6;12(1):e001484 doi: 10.1136/lupus-2024-001484
Connelly, et al. use Delphi methods to achieve consensus to include eight domains of active disease to define treatment response in a novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE).
Outcomes of patients with systemic lupus erythematosus treated with belimumab: a post hoc efficacy analysis of five phase III clinical trials by British Isles Lupus Assessment Group-based Combined Lupus Assessment criteria
RMD Open 2025;11:e005-444 DOI:10.1136/rmdopen-2025-005444
Padrodis et al. aimed to determine belimumab efficacy assessed using BICLA in patients with SLE included in the phase III belimumab RCTs. The benefit of belimumab was found to be more prominent when combined with anti-malarial agents. Furthermore, using BICLA, the authors validated the results from foundational trials originally assessing belimumab efficacy using SLE Responder Index 4 thus, corroborating the efficacy of #belimumab in SLE.
Telitacicept in Patients with Active Systemic Lupus Erythematosus: Results of A Phase 2b, Randomised, Double-blind, Placebo-controlled Trial
Ann Rheum Dis. 2023; DOI: 10.1136/ard-2023-224854
This Phase 2 trial demonstrated the efficacy and acceptable safety profile of telitacicept in patients with SLE. The safety profile of telitacicept was comparable with that observed in clinical trials of other B cell-targeting agents.
Keywords:
Association Between Severe Nonadherence to Hydroxychloroquine and Systemic Lupus Erythematosus Flares, Damage, and Mortality in 660 Patients From the SLICC Inception Cohort
Arthritis Rheumatol. 2023; 75(12):2195–2206 DOI: 10.1002/art.42645
n this study, severe nonadherence to hydroxychloroquine (HCQ) was independently associated with the risk of SLE flare in the following year, early damage and 5-year mortality.
Keywords:
EULAR Recommendations for the Management of Systemic Lupus Erythematosus: 2023 Update
Ann Rheum Dis 2023;83(1):15–29 DOI: 10.1136/ard-2023-224762
The objective of this international task force was to update the EULAR recommendations for the management of SLE. The Task Force agreed on 5 overarching principles and 13 recommendations, generating an overall framework for the approach to a patient with SLE. The updated recommendations provide consensus guidance on the management of SLE, combining evidence and expert opinion.
Keywords:
Sustained Glucocorticoid Tapering in the Phase 3 Trials of Anifrolumab: A post hoc Analysis of the TULIP-1 and TULIP-2 Trials
Rheumatology (Oxford). 2023 doi: 10.1093/rheumatology/keac491
Pooled analysis of the TULIP trials demonstrates that sustained glucocorticoid (GC) tapering is associated with several clinical benefits in patients with moderate-to-severe SLE.
Keywords:
Assessing the Costs of Neuropsychiatric Disease in the Systemic Lupus International Collaborating Clinics (SLICC) Cohort using Multistate Modelling
Arthritis Care Res (Hoboken). 2023 doi: 10.1002/acr.25090.
First study to assess the long-term economic burden of neurologic and/or psychiatric (NP) lupus in an international, multi-ethnic inception cohort, concludes that patients with new/ongoing SLE or non-SLE NP events incurred higher direct and indirect costs.
Trial of Anti-BDCA2 Antibody Litifilimab for Systemic Lupus Erythematosus
N Engl J Med. 2022;387(10):894–904 doi: 10.1056/NEJMoa2118025
Phase 2 study, in patients with systemic lupus erythematosus, shows that litifilimab is associated with a greater reduction from baseline in the number of swollen and tender joints than placebo, over a period of 24 weeks.