Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
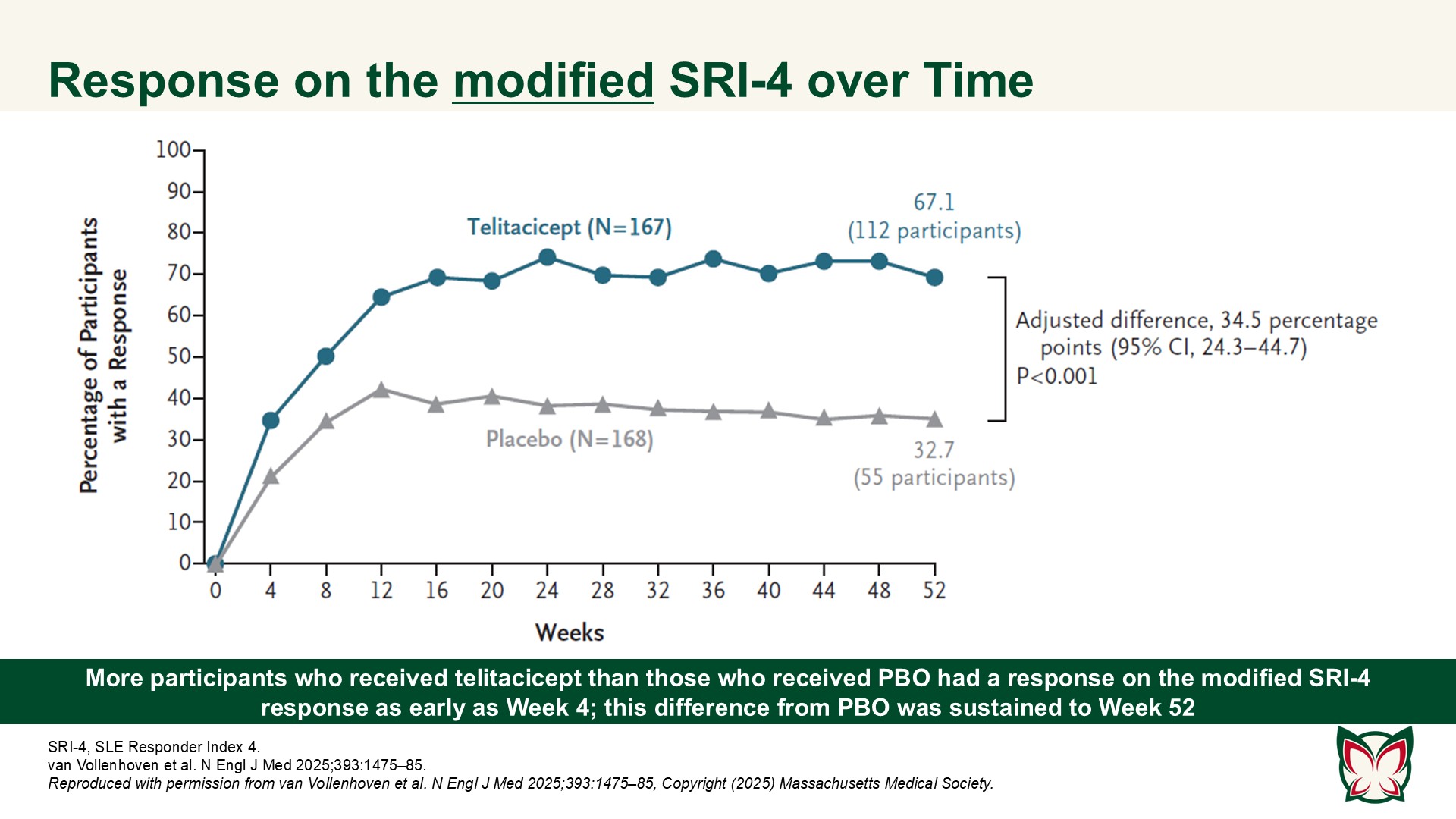
A Phase 3 trial of telitacicept for systemic lupus erythematosus
N Engl J Med 2025;393:1475-85 Doi: 10.1056/NEJMoa2414719
In this 52-week trial involving participants with active SLE who were receiving background therapy, the incidence of a clinical response was higher with telitacicept than with PBO. van Vollenhoven et al. report efficacy and safety results of a Phase 3 trial of telitacicept at a dose of 160mg weekly as compared with PBO in Chinese persons with active SLE.
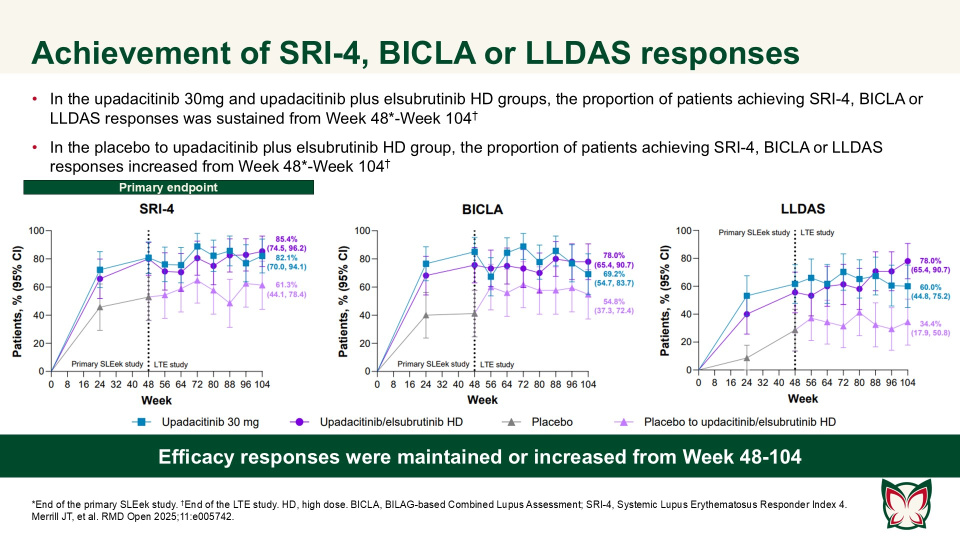
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
Cenerimod, a sphingosine-1-phosphate receptor modulator, versus placebo in patients with moderate-to-severe systemic lupus erythematosus (CARE): an international, double-blind, randomised, placebo-controlled, phase 2 trial
Lancet Rheumatol. 2024. Epub ahead of print. DOI: 10.1016/S2665-9913(24)00246-7
Askanase et al. assessed the efficacy, safety, and tolerability of cenerimod in patients with moderate-to-severe SLE. While the primary endpoint of reducing mSLEDAI-2K scores at Month 6 was not achieved, cenerimod 4.0mg showed a significant reduction in disease activity versus placebo. Adverse events, including lymphopenia, were dose-dependent but manageable, and overall treatment was well tolerated.
Effect of iberdomide on cutaneous manifestations in systemic lupus erythematosus: a randomized phase 2 clinical trial
JAAD. 2024. Epub ahead of print DOI: 10.1016/j.jaad.2024.09.074
Werth et al. demonstrated that iberdomide significantly improved cutaneous lupus erythematosus (CLE) outcomes, particularly in subacute and chronic CLE patients, by reducing Cutaneous Lupus Area and Severity Index Activity (CLASI-A) scores. The study showed continued efficacy through 24 weeks, with the 0.45 mg dose providing the greatest improvement in patients with severe baseline scores, and iberdomide was well-tolerated over 104 weeks.
Keywords:
Efficacy and safety of upadacitinib or elsubrutinib alone or in combination for systemic lupus erythematosus: A Phase 2 randomized controlled trial
Arthritis Rheumatol 2024 DOI: 10.1002/art.42926 Epub ahead of print
Daily oral upadacitinib 30 mg and ABBV-599 high dose (elsubrutinib 60 mg QD + upadacitinib 30 mg) were effective in multiple outcome measures including disease activity, flares, time to first flare, and joint counts.
Keywords:
Association Between Severe Nonadherence to Hydroxychloroquine and Systemic Lupus Erythematosus Flares, Damage, and Mortality in 660 Patients From the SLICC Inception Cohort
Arthritis Rheumatol. 2023; 75(12):2195–2206 DOI: 10.1002/art.42645
n this study, severe nonadherence to hydroxychloroquine (HCQ) was independently associated with the risk of SLE flare in the following year, early damage and 5-year mortality.
Keywords:
Telitacicept in Patients with Active Systemic Lupus Erythematosus: Results of A Phase 2b, Randomised, Double-blind, Placebo-controlled Trial
Ann Rheum Dis. 2023; DOI: 10.1136/ard-2023-224854
This Phase 2 trial demonstrated the efficacy and acceptable safety profile of telitacicept in patients with SLE. The safety profile of telitacicept was comparable with that observed in clinical trials of other B cell-targeting agents.
Keywords:
Machine Learning Identifies Clusters of Longitudinal Autoantibody Profiles Predictive of Systemic Lupus Erythematosus Disease Outcomes
Ann Rheum Dis 2023;82(7):927–36 doi 10.1136/ard-2022-223808
Choi, et al. used machine clustering techniques to divide SLE patients into four distinct clusters. This could potentially be used to predict future clinical outcomes, and as benchmarks to study other SLE-related outcomes.
Keywords:
Evaluation of the LFA-REAL Clinician-reported Outcome (ClinRO) and Patient-reported Outcome (PRO): Prespecified Analysis of the Phase III Ustekinumab Trial in Patients with SLE
Lupus Sci Med. 2023 doi:10.1136/lupus-2022-000875
The Lupus Foundation of America Rapid Evaluation of Activity in Lupus (LFA-REAL) clinician-reported outcome (ClinRO) and patient-reported outcome (PRO) systems show potential as a flexible resource in the evaluation of lupus disease activity and a simple, user-friendly outcome measure for SLE studies.
Keywords:
Assessing the Costs of Neuropsychiatric Disease in the Systemic Lupus International Collaborating Clinics (SLICC) Cohort using Multistate Modelling
Arthritis Care Res (Hoboken). 2023 doi: 10.1002/acr.25090.
First study to assess the long-term economic burden of neurologic and/or psychiatric (NP) lupus in an international, multi-ethnic inception cohort, concludes that patients with new/ongoing SLE or non-SLE NP events incurred higher direct and indirect costs.