Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
Secukinumab in active lupus nephritis: Results from Phase III, randomised, placebo controlled study (SELUNE) and open-label extension study
Rheumatology 2025 Doi: 10.1093/rheumatology/keaf536 Epub ahead of print
Secukinumab did not demonstrate superior efficacy over PBO in patients with active LN; secukinumab was well-tolerated with no new or unexpected safety signals detected. A Phase-III core study (SELUNE) and an extension study, were conducted by Zhao et al. to evaluate the efficacy and safety of SC secukinumab 300mg compared with PBO, in combination with the SoC, in patients with active LN.
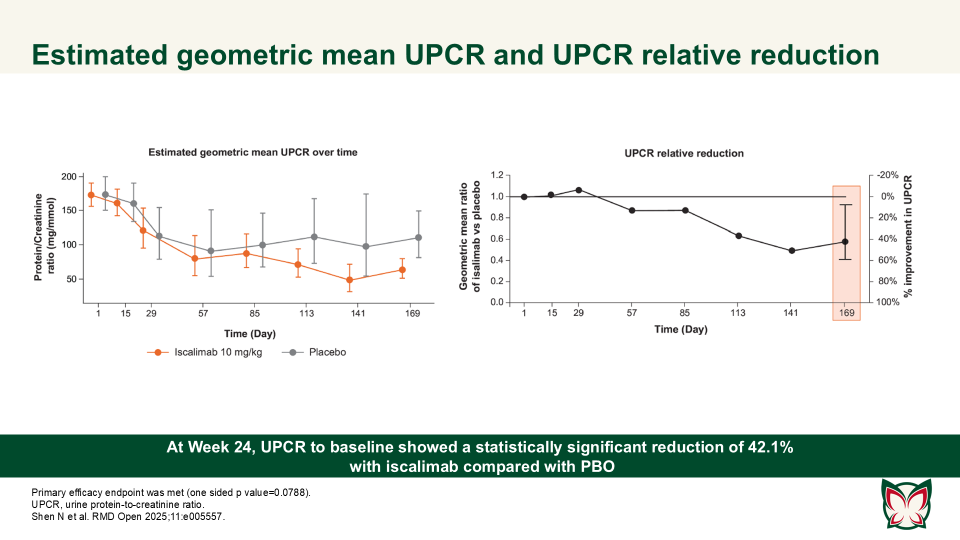
Efficacy, pharmacokinetics and safety of iscalimab (CFZ533) in patients with proliferative lupus nephritis: A randomised, double-blind, placebo-controlled, Phase II study
RMD Open 2025;11:e005557 Doi:10.1136/rmdopen-2025-005557
Shen N et al. report that iscalimab was clinically effective and generally well tolerated; in addition, it was devoid of the thromboembolic risk, characteristic of Fc active anti-CD40L therapies.
BCMA-targeted CAR T cell therapy can effectively induce disease remission in refractory lupus nephritis
Ann Rheum Dis 2025;0:1−9
Hu et al. report that anti-BCMA only CAR T cell can help LN patients safely and effectively, indicating its potential to be a feasible therapeutic strategy in treating autoimmune diseases with abnormal humoral immune responses.
Effect of long-term voclosporin treatment on renal histology in patients with active lupus nephritis with repeat renal biopsies
Arthritis & Rheumatology 2025; 0:1–7 doi: 10.1002/art.43209
Exposure to voclosporin for a median of 18 months was not associated with onset or progression of nephrotoxicity based on evaluation of histologic compartments and vascular lesions. Rovin et al. characterised the impact of voclosporin on kidney histology in patients with LN who had protocolized repeat kidney biopsies in the AURORA clinical trials.
Keywords:
Efficacy and safety of obinutuzumab in active lupus nephritis
NEJM, 2025. Epub ahead of print. DOI: 10.1056/NEJMoa2410965
Furie et al. demonstrated that obinutuzumab plus standard therapy significantly improved complete renal response at Wk76 compared with placebo. No unexpected safety signals were identified, though infections and COVID-19-related events were more frequent in the obinutuzumab group.
Belimumab versus telitacicept in sequential treatment after rituximab for refractory lupus nephritis: A real-world multicentre study
Lupus Science & Medicine, 2025;12:e001296 DOI:10.1136/lupus-2024-001296
Chen et al. demonstrated that sequential treatment with belimumab or telitacicept following rituximab (RTX) is a potential therapeutic approach for treating refractory LN. Major AEs included immunoglobin deficiency, respiratory tract infections and urinary tract infections, which are consistent with previous studies.
Comparison of a voclosporin-based triple immunosuppressive therapy to high-dose glucocorticoid-based immunosuppressive therapy: A propensity analysis of the AURA-LV and AURORA 1 studies and ALMS
Lupus Science & Medicine 2024;11:e001319 DOI: 10.1136/lupus-2024-001319
Dall’Era et al. conducted a propensity analysis to compare voclosporin-based triple immunosuppressive therapy with high-dose GC-based regimens for active LN. Voclosporin showed fewer AEs, improved safety, and significantly reduced proteinuria over six months, suggesting a superior risk-benefit profile for patients with lupus nephritis.
Keywords:
Urinary soluble CD163 is useful as "liquid biopsy" marker in lupus nephritis at both diagnosis and follow-up to predict impending flares
J Transl Autoimmun 2024;9:100244 DOI 10.1016/j.jtauto.2024.100244
Renaudineau, et al. show that the urinary sCD163/creatinurea ratio is a parameter than can be used in addition to anti-dsDNA antibodies, anti-C1q antibodies, C3 complement fraction, the protein excretion to creatinine ratio and the estimated glomerular filtration rate.
Keywords:
Immunosuppressives discontinuation after renal response in lupus nephritis: predictors of flares, time to withdrawal and long-term outcomes
Rheumatol 2024 DOI 10.1093/rheumatology/keae381 Epub ahead of print
This study by Panagiotopoulos, et al. showed that an early complete renal response achievement, persistent hydroxychloroquine use, and the maintenance of optimal low disease activity during follow-up in immunosuppressive (IS) tapering and discontinuation are fundamental in LN treatment. The authors also found that long-term renal outcomes are mainly associated with renal flares during IS tapering.
Combination of anti-SSA/Ro60 and anti-dsDNA serotype is predictive of belimumab renal response in patients with lupus nephritis
Lupus Sci Med. 2024 May 28;11(1) doi: 10.1136/lupus-2024-001156
The combination of anti-dsDNA and anti-SSA/Ro60 serotype may help to foretell the patient’s renal response to belimumab. Investigators here aimed to explore the effectiveness of belimumab on active LN, exploring predictors, including serological biomarkers, of renal response to belimumab in a real-world setting.