Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
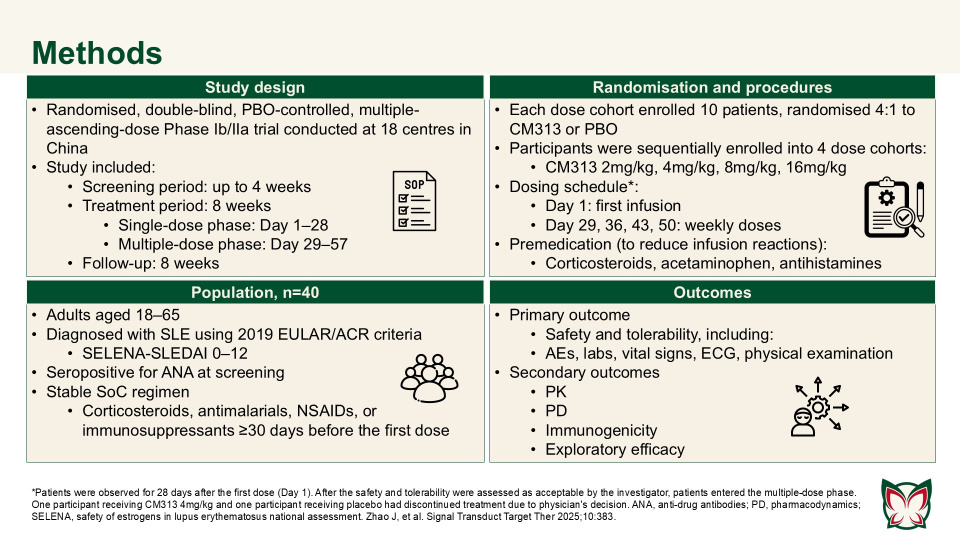
Anti-CD38 monoclonal antibody CM313 for systemic lupus erythematosus: A randomized, double-blind, placebo-controlled Phase Ib/IIa trial
Signal Transduct Target Ther 2025;10:383 Doi: 10.1038/s41392-025-02487-2
Zhao et al. showed that CM313 was well tolerated in adult patients with SLE at doses of 2–16mg/kg and showed encouraging pharmacodynamic effects and preliminary efficacy at doses of 8 and 16 mg/kg QW. CM313 also produced dose-dependent and clinically meaningful improvements in key serological biomarkers of SLE.
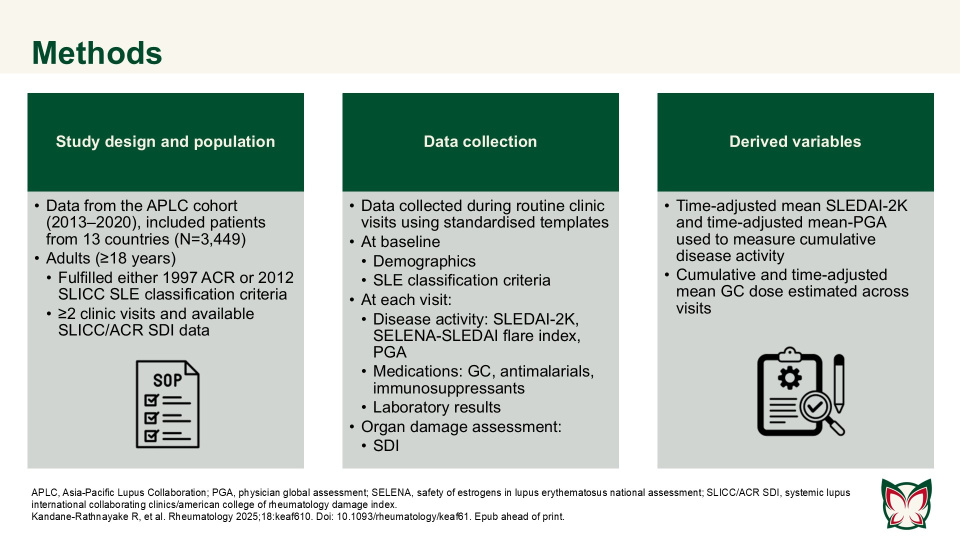
Predictors of damage accrual by organ domain in systemic lupus erythematosus
Rheumatology 2025;18:keaf610 Doi: 10.1093/rheumatology/keaf610 Epub ahead of print
Kandane-Rathnayake et al. reported that risk factors for individual organ system damage were highly varied in patients with SLE, and not all factors associated with domain-specific damage were captured by summed systemic lupus international collaborating clinics/american college of rheumatology damage index (SLICC/ACR SDI) for overall organ damage.
Efficacy and safety of allogeneic CD19 CAR NK-cell therapy in systemic lupus erythematosus: A case series in China
Lancet 2025 Doi:10.1016/S0140-6736(25)01671-X Epub ahead of print
Gao et al. report that allogeneic CAR NK-cell therapy is a potent option for treatment of autoimmune diseases and may address limitations of current autologous CAR T-cell therapy, including manufacturing scale and time, access, safety, and cost. Authors evaluated the safety, tolerability, and efficacy of allogeneic CD19 CAR NK-cell therapy in patients with relapsed or refractory SLE.
Keywords:
Low-dose belimumab reduced risk of flares in patients with systemic lupus erythematosus: A multicentre, randomised, double-blind, placebo-controlled trial
Ann Rheum Dis 2025 Doi: 10.1016/j.ard.2025.10.010. Epub ahead of print
Sun et al. provides the first RCT evidence that low-dose belimumab reduces flares in patients with SLE with low-grade disease activity. Authors evaluated the efficacy of low-dose belimumab for disease flare prevention in Chinese patients with low-grade SLE (SELENA-SLEDAI ≤6).
Keywords:
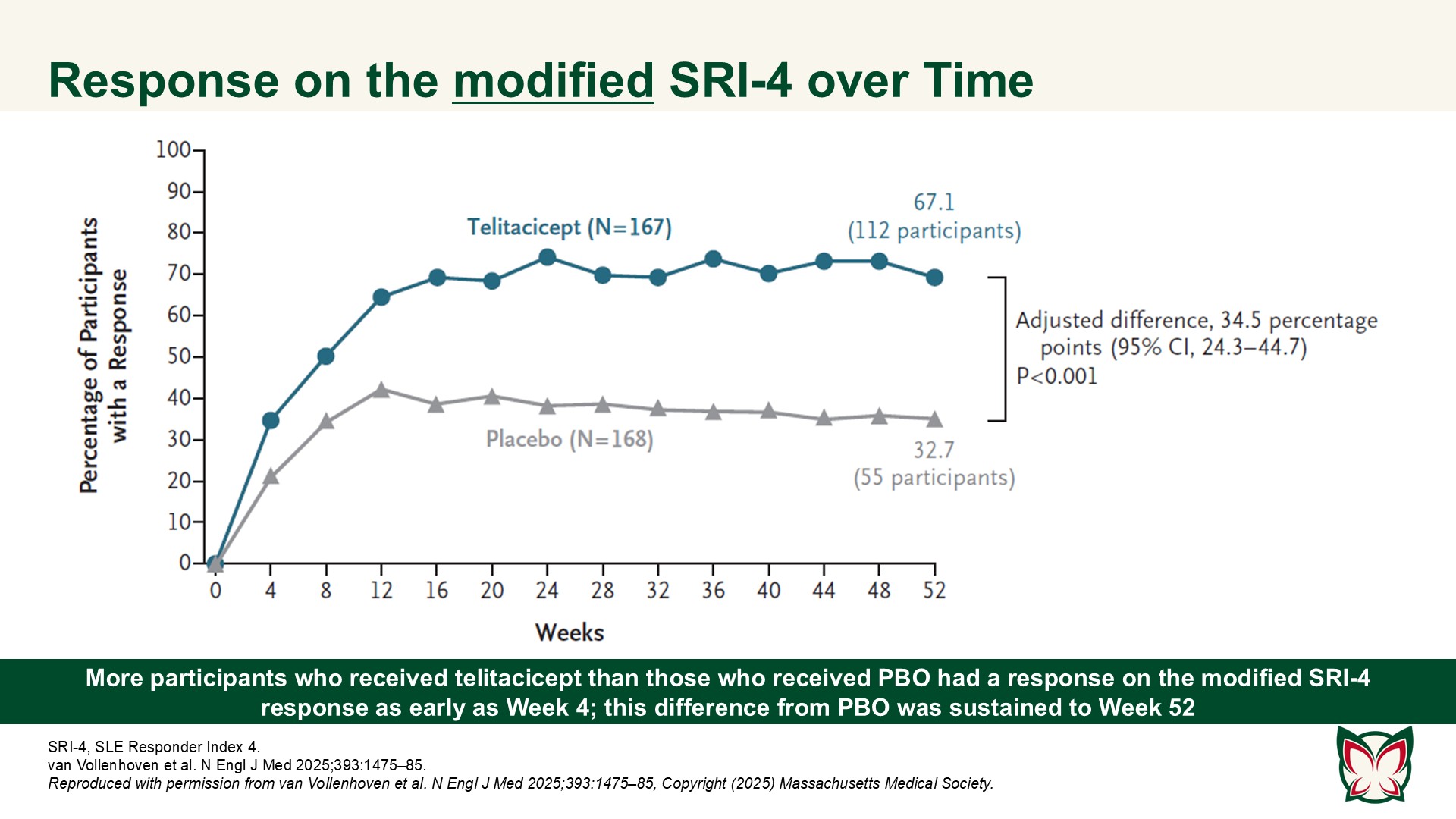
A Phase 3 trial of telitacicept for systemic lupus erythematosus
N Engl J Med 2025;393:1475-85 Doi: 10.1056/NEJMoa2414719
In this 52-week trial involving participants with active SLE who were receiving background therapy, the incidence of a clinical response was higher with telitacicept than with PBO. van Vollenhoven et al. report efficacy and safety results of a Phase 3 trial of telitacicept at a dose of 160mg weekly as compared with PBO in Chinese persons with active SLE.
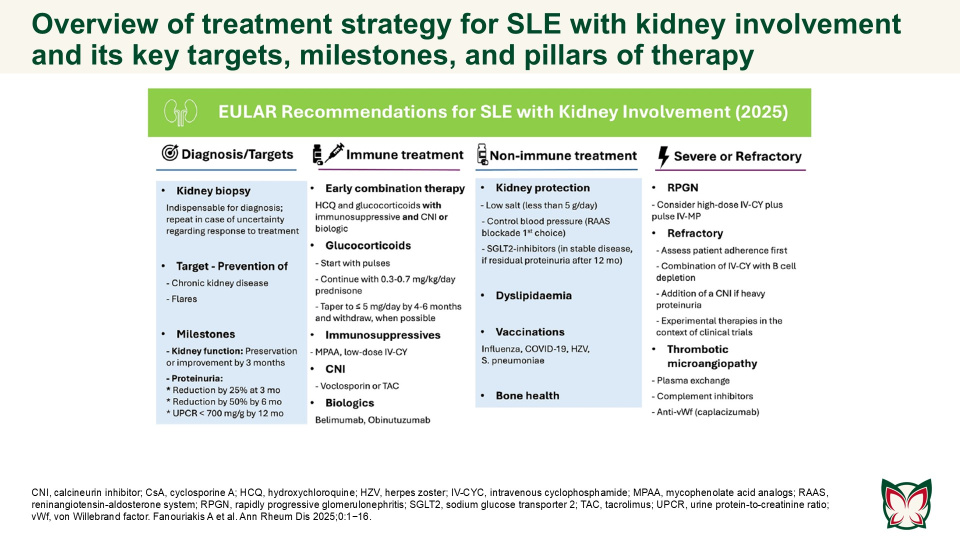
EULAR recommendations for the management of systemic lupus erythematosus with kidney involvement: 2025 update
Ann Rheum Dis 2025;0:1−16 Doi: 10.1016/j.ard.2025.09.007
The updated EULAR recommendations provide evidence- and expert-based consensus on the management of SLE with kidney involvement, adjusted for severity, and taking into consideration long-term efficacy, safety, cost, and local availability of drugs. Fanouriakis A et al. updated the 2019 EULAR/ ERA-EDTA recommendations for the management of SLE with kidney involvement, taking into consideration emerging evidence and recent developments in the field.
Keywords:
Secukinumab in active lupus nephritis: Results from Phase III, randomised, placebo controlled study (SELUNE) and open-label extension study
Rheumatology 2025 Doi: 10.1093/rheumatology/keaf536 Epub ahead of print
Secukinumab did not demonstrate superior efficacy over PBO in patients with active LN; secukinumab was well-tolerated with no new or unexpected safety signals detected. A Phase-III core study (SELUNE) and an extension study, were conducted by Zhao et al. to evaluate the efficacy and safety of SC secukinumab 300mg compared with PBO, in combination with the SoC, in patients with active LN.
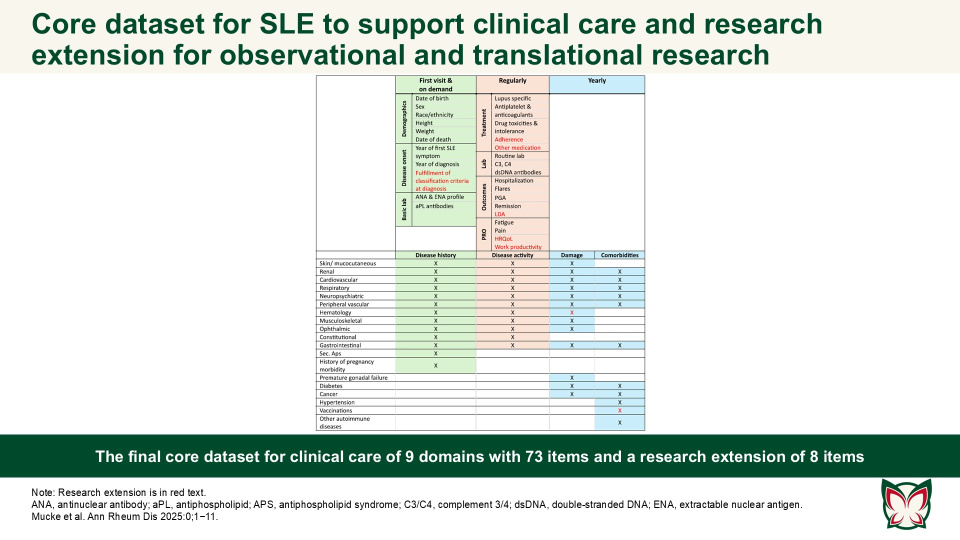
EULAR recommendations for a core dataset to support clinical care and translational and observational research in systemic lupus erythematosus
Ann Rheum Dis 2025:0;1−11 Doi: 10.1016/j.ard.2025.07.001
The presented clinical core dataset and its research extension are designed to improve SLE patient care and facilitate collaborative research by ensuring the comparability of datasets and cohort descriptions. The aim of this EULAR taskforce by Mucke et al. was to define a core set of essential items for the comprehensive care of SLE patients in clinical practice, with an extension for vital elements required for translational and observational research.
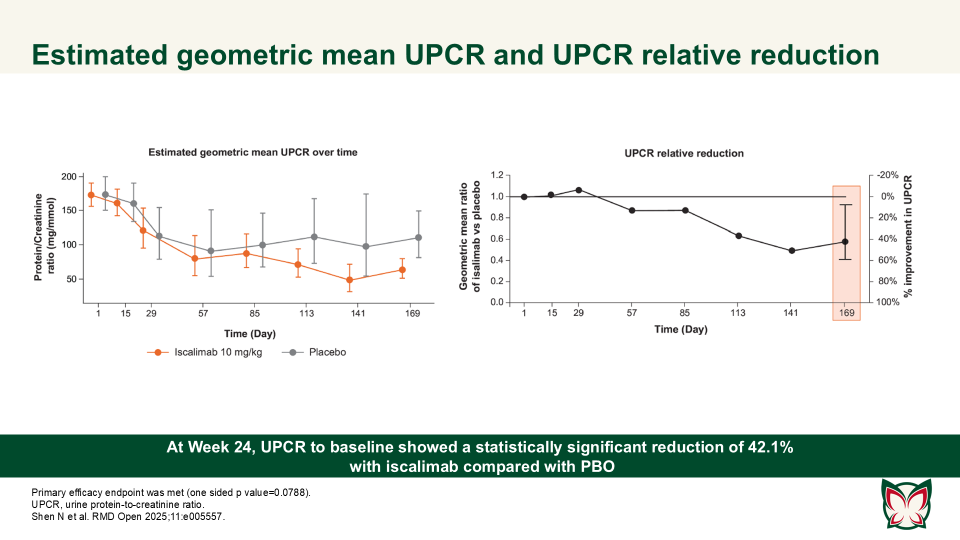
Efficacy, pharmacokinetics and safety of iscalimab (CFZ533) in patients with proliferative lupus nephritis: A randomised, double-blind, placebo-controlled, Phase II study
RMD Open 2025;11:e005557 Doi:10.1136/rmdopen-2025-005557
Shen N et al. report that iscalimab was clinically effective and generally well tolerated; in addition, it was devoid of the thromboembolic risk, characteristic of Fc active anti-CD40L therapies.
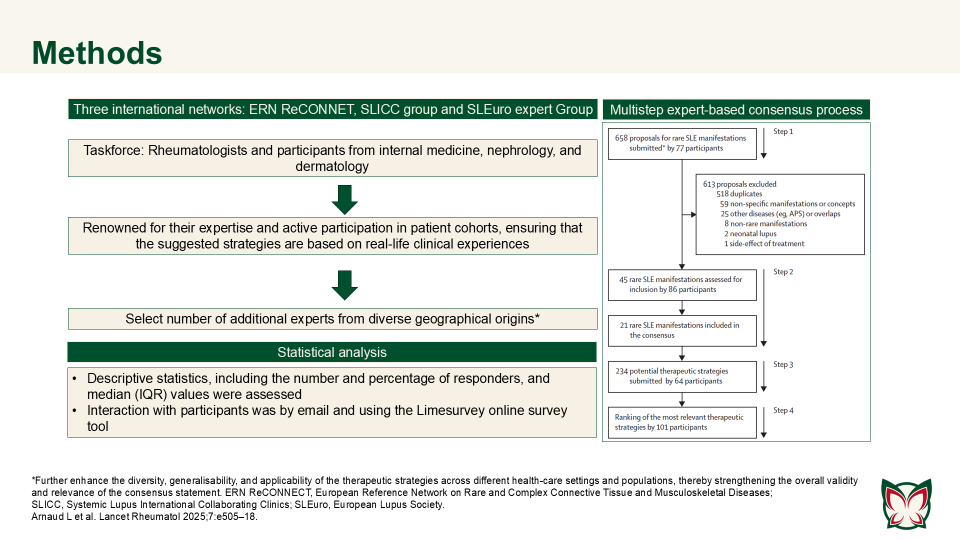
ERN ReCONNET–SLICC–SLEuro expert consensus on the therapeutic management of rare systemic lupus erythematosus manifestations
Lancet Rheumatol 2025;7:e505–18 Doi: 10.1016/S2665-9913(25)00063-3
This systematic review highlighted the major gaps in evidence regarding rare SLE manifestations, with many findings dependent on variable methodologies or single reports. Arnard et al. established an international consensus on therapeutic strategies for rare SLE manifestations.