Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
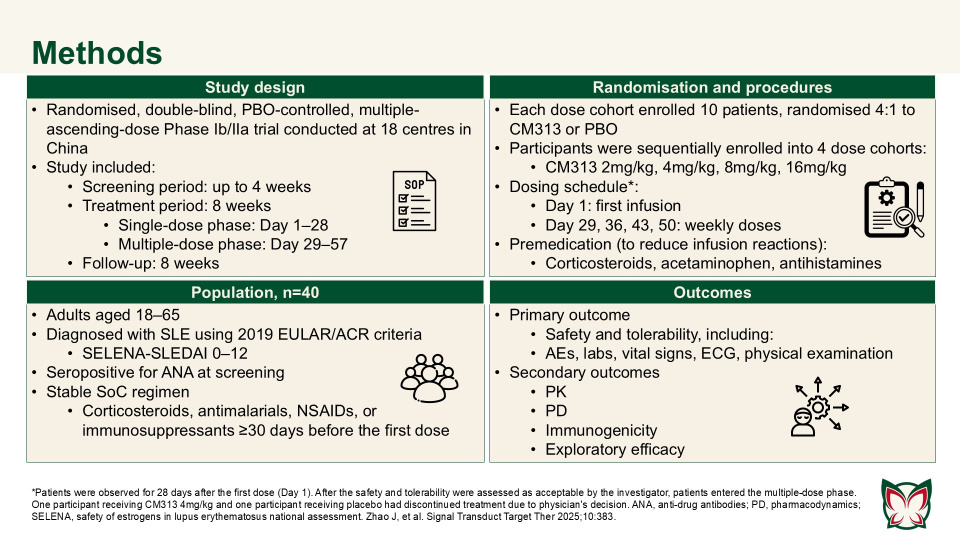
Anti-CD38 monoclonal antibody CM313 for systemic lupus erythematosus: A randomized, double-blind, placebo-controlled Phase Ib/IIa trial
Signal Transduct Target Ther 2025;10:383 Doi: 10.1038/s41392-025-02487-2
Zhao et al. showed that CM313 was well tolerated in adult patients with SLE at doses of 2–16mg/kg and showed encouraging pharmacodynamic effects and preliminary efficacy at doses of 8 and 16 mg/kg QW. CM313 also produced dose-dependent and clinically meaningful improvements in key serological biomarkers of SLE.
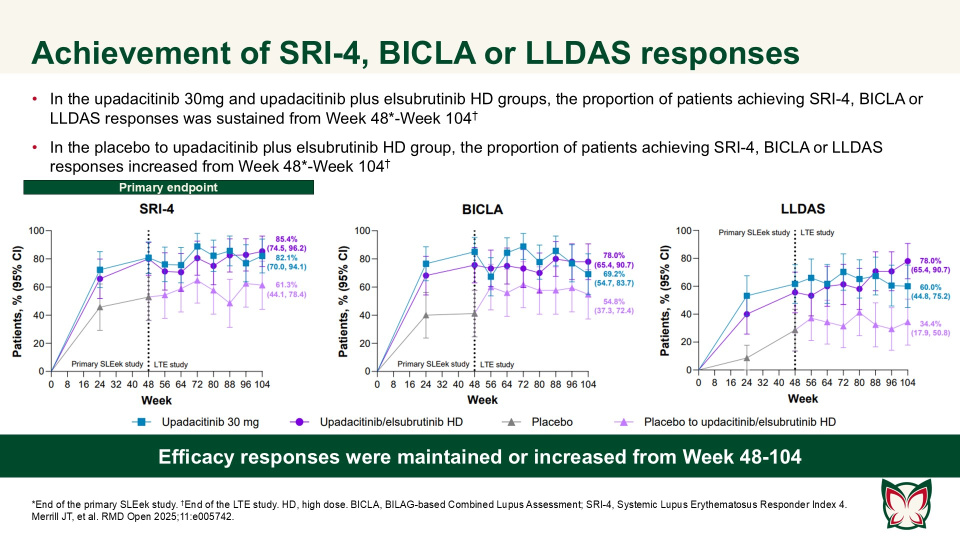
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
Efficacy and safety of upadacitinib or elsubrutinib alone or in combination for systemic lupus erythematosus: A Phase 2 randomized controlled trial
Arthritis Rheumatol 2024 DOI: 10.1002/art.42926 Epub ahead of print
Daily oral upadacitinib 30 mg and ABBV-599 high dose (elsubrutinib 60 mg QD + upadacitinib 30 mg) were effective in multiple outcome measures including disease activity, flares, time to first flare, and joint counts.