Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
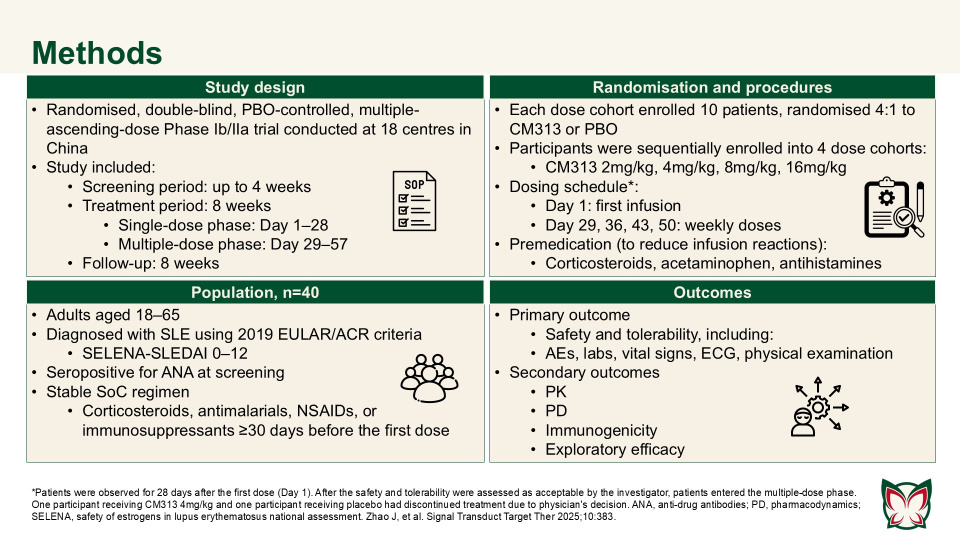
Anti-CD38 monoclonal antibody CM313 for systemic lupus erythematosus: A randomized, double-blind, placebo-controlled Phase Ib/IIa trial
Signal Transduct Target Ther 2025;10:383 Doi: 10.1038/s41392-025-02487-2
Zhao et al. showed that CM313 was well tolerated in adult patients with SLE at doses of 2–16mg/kg and showed encouraging pharmacodynamic effects and preliminary efficacy at doses of 8 and 16 mg/kg QW. CM313 also produced dose-dependent and clinically meaningful improvements in key serological biomarkers of SLE.
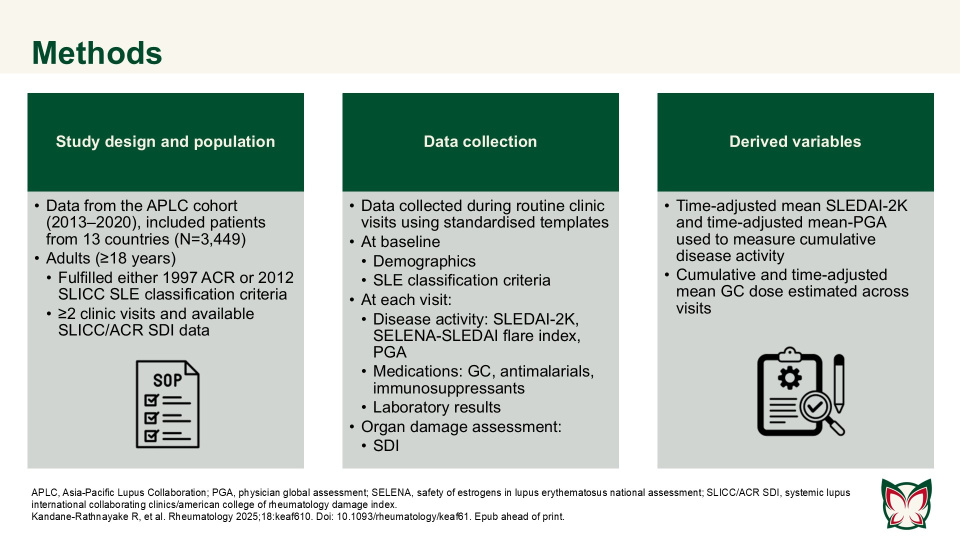
Predictors of damage accrual by organ domain in systemic lupus erythematosus
Rheumatology 2025;18:keaf610 Doi: 10.1093/rheumatology/keaf610 Epub ahead of print
Kandane-Rathnayake et al. reported that risk factors for individual organ system damage were highly varied in patients with SLE, and not all factors associated with domain-specific damage were captured by summed systemic lupus international collaborating clinics/american college of rheumatology damage index (SLICC/ACR SDI) for overall organ damage.
Association of lupus low disease activity state and remission with reduced organ damage and flare in systemic lupus erythematosus patients with high disease activity
Rheumatology 2024; Epub ahead of print DOI: 10.1093/rheumatology/keae631
Kandane-Rathnayake et al. demonstrated that achieving Lupus Low Disease Activity State (LLDAS) or remission in patients with high disease activity status (HDAS) significantly reduces the risk of organ damage accrual and flares. However, HDAS was found to be a poor prognostic indicator as fewer patients with HDAS attained and sustained LLDAS or remission when compared with non-HDAS patients.
Keywords:
Association of sustained lupus low disease activity state with improved outcomes in systemic lupus erythematosus: a multinational prospective cohort study
Lancet Rheumatol 2024:S2665-9913(24)00121-8 DOI 10.1016/S2665-9913(24)00121-8 Epub ahead of print
This study by Golder, et al. showed a significant protective association of lupus low disease activity state (LLDAS) and remission against damage accrual and flare. The authors also found a threshold of 3 months sustained LLDAS or remission, and that 3 months of sustained LLDAS are attainable in the setting of a 6–12-month clinical trial.
Keywords:
Risk of flare and damage accrual after tapering glucocorticoids in modified serologically active clinically quiescent patients with systemic lupus erythematosus: A multinational observational cohort study
Ann Rheum Dis. 2024 Feb 29:ard-2023-225369 doi: 10.1136/ard-2023-225369 Epub ahead of print
Flare risk did not increase following glucocorticoid tapering in modified serologically active clinically quiescent patients with SLE. They also found that antimalarial use was associated with decreased flare risk.
Impact of Low Disease Activity, Remission, and Complete Remission on Flares Following Tapering of Corticosteroids and Immunosuppressive Therapy in Patients with Systemic Lupus Erythematous: A Multinational Cohort Study
The Lancet Rheumatology, 2023, Volume 5, Issue 10, e584 - e593 DOI: https://doi.org/10.1016/S2665-9913(23)00209-6
In this study, tapering of corticosteroids or immunosuppressive therapy in patients in LLDAS, remission, or complete remission was associated with excess flares versus continuing with therapy. Tapering in complete remission was associated with lower odds of flares compared with tapering in LLDAS or remission. In addition, patients with longer sustained duration of LLDAS or remission at the time of tapering had lower odds of flare and longer time to flare versus those with a shorter duration of LLDAS or remission.
Baricitinib for Systemic Lupus Erythematosus: a Double-blind, Randomised, Placebo-controlled, Phase 3 trial (SLE-BRAVE-II)
Lancet. 2023 doi: 10.1016/S0140-6736(22)02546-6
Negative results of SLE-BRAVE-II trial show that evidence for the efficacy of baricitinib in SLE is inconclusive.
Lupus Low Disease Activity State and Remission and Risk of Mortality in Patients with Systemic Lupus Erythematosus: A Prospective, Multinational, Longitudinal Cohort Study
Lancet Rheumatol. 2022. Epub ahead of print. doi: 10.1016/S2665-9913(22)00304-6
Lupus low disease activity state (LLDAS) significantly reduced the risk of mortality in patients with SLE.