Publications
Find coverage of the latest original articles on Lupus, focusing on those with data on therapeutic interventions and those that have clinical impact.
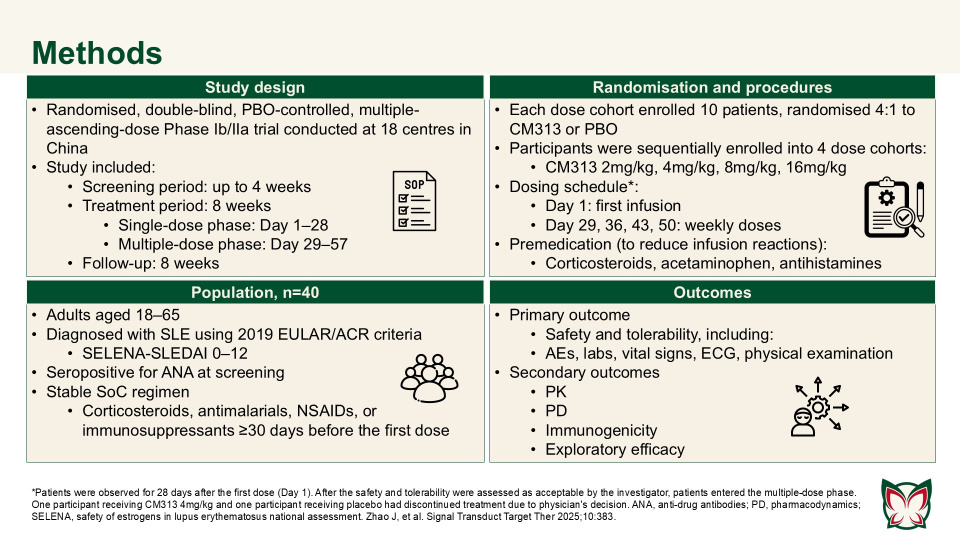
Anti-CD38 monoclonal antibody CM313 for systemic lupus erythematosus: A randomized, double-blind, placebo-controlled Phase Ib/IIa trial
Signal Transduct Target Ther 2025;10:383 Doi: 10.1038/s41392-025-02487-2
Zhao et al. showed that CM313 was well tolerated in adult patients with SLE at doses of 2–16mg/kg and showed encouraging pharmacodynamic effects and preliminary efficacy at doses of 8 and 16 mg/kg QW. CM313 also produced dose-dependent and clinically meaningful improvements in key serological biomarkers of SLE.
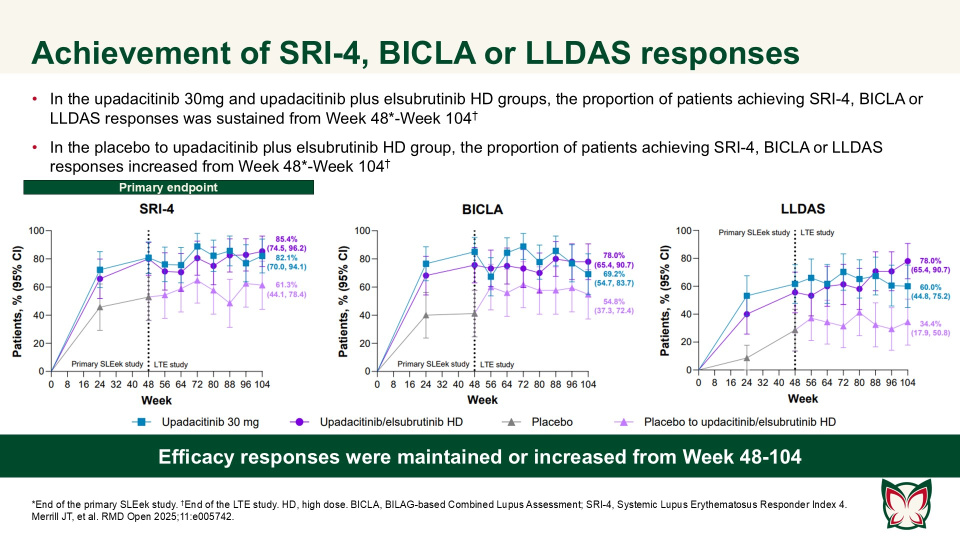
Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: results through 104 weeks in a long-term extension study
RMD Open 2025;11:e005742 Doi:10.1136/rmdopen-2025-005742
In this long-term extension of the Phase 2 SLEek study, Merrill et al. report that through an additional 56-weeks of treatment, upadacitinib as monotherapy or combined with elsubrutinib demonstrated sustained or improved efficacy in multiple endpoints in patients with moderately to severely active SLE.
Keywords:
LLDAS and remission attainment with anifrolumab treatment in patients with systemic lupus erythematosus: results from the TULIP and long-term extension randomised controlled trials
Ann Rheum Dis. 2025:S0003-496700071-8. DOI: 10.1016/j.ard.2025.01.016. Epub ahead of print
Morand et al. conducted a post-hoc analysis of the phase III TULIP-1 and TULIP-2 trials and their long-term extension, including 369 patients with moderate to severe SLE, to evaluate the long-term impact of anifrolumab on attainment of LLDAS and DORIS-defined remission. The results demonstrated that anifrolumab significantly improved the likelihood, speed, and duration of LLDAS and DORIS remission versus placebo over 4 years, with benefits sustained throughout the treatment period.
Keywords:
Evolution and trajectory of B-cell targeted therapies in rheumatic diseases
Lancet Rheumatol, 2025. Epub ahead of print
Carter et al. review the clinical and mechanistic development of B-cell targeted therapies over the last two decades in autoimmune rheumatic diseases. B-cell depletion depth, repopulation dynamics, and immunogenicity determine long-term efficacy and inform the rationale for emerging
dual-targeted approaches, particularly in systemic lupus erythematosus where belimumab and rituximab combinations show potential to mitigate relapse driven by BAFF.
Opportunities and limitations of B bell depletion approaches in SLE
Nature Review Rheumatol, 2025;21:111–126 DOI: 10.1038/s41584-024-01210-9
Stockfelt et al. reviewed the long-term efficacy and challenges of B cell depletion strategies in SLE. Rituximab, a CD20-targeting monoclonal antibody, has demonstrated efficacy in a subset of patients but remains limited by immunogenicity, residual B cells, and B-cell activating factor (BAFF)-mediated relapse. Newer strategies incorporating CAR T cells, bispecific T cell engagers, and combination therapies aim to enhance B cell depletion and optimise outcomes.
Keywords:
CD19-CAR T-cell therapy induces deep tissue depletion of B cells
Ann Rheum Dis 2024;0:1–8 DOI 10.1136/ard-2024-226142
Tur et al. demonstrated that CD19-targeting CAR T-cell therapy results in the depletion of B cells within deep tissues. The study highlights significant reductions in pathogenic B-cell populations, particularly in autoimmune diseases, after CD19-CAR T-cell administration.
Keywords:
Assessment and Personalised Advice for Fatigue in Systemic Lupus Erythematosus Using an Innovative Digital Tool: The Lupus Expert System for the Assessment of Fatigue (LEAF) Study
RMD open. 2023; DOI:10.1136/rmdopen-2023-003476
Kawka et al investigated the use of the LEAF digital health tool to successfully assess the extent of patient-reported fatigue in SLE patients.
Kidney Outcomes and Preservation of Kidney Function with Obinutuzumab in Patients with Lupus Nephritis: A Post Hoc Analysis of the NOBILITY Trial
Arthritis Rheumatol. 2023 DOI 10.1002/art.42734
This post hoc analysis of the Phase 2 NOBILITY trial determined that obinutuzumab plus standard of care improved the likelihood of long-term preservation of kidney function and improved kidney function with less glucocorticoid use in patients with lupus nephritis.
Keywords:
Impact of Low Disease Activity, Remission, and Complete Remission on Flares Following Tapering of Corticosteroids and Immunosuppressive Therapy in Patients with Systemic Lupus Erythematous: A Multinational Cohort Study
The Lancet Rheumatology, 2023, Volume 5, Issue 10, e584 - e593 DOI: https://doi.org/10.1016/S2665-9913(23)00209-6
In this study, tapering of corticosteroids or immunosuppressive therapy in patients in LLDAS, remission, or complete remission was associated with excess flares versus continuing with therapy. Tapering in complete remission was associated with lower odds of flares compared with tapering in LLDAS or remission. In addition, patients with longer sustained duration of LLDAS or remission at the time of tapering had lower odds of flare and longer time to flare versus those with a shorter duration of LLDAS or remission.
Health Related Quality of Life, Remission and Low Lupus Disease Activity State in Patients with Systemic Lupus Erythematosus
Rheumatology (Oxford). 2023 doi: 10.1093/rheumatology/kead407 Epub ahead of print
One of the first studies to investigate associations between disease activity and QoL, using validated generic and disease-specific PRO questionnaires, found that DORIS remission and LLDAS were associated with significant and clinically relevant higher QoL in most PRO domains of the LupusPRO (disease specific) and SF-36 (generic) questionnaires, but not with LupusQoL and SLEQOL disease-specific questionnaires.